10.6 Basic ERCP Technique 1. Inserting The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papilla with Separate Bile and Pancreatic Duct Orifices
JOP. J Pancreas (Online) 2013 May 10; 14(3):302-303. MULTIMEDIA ARTICLE – Clinical Imaging Papilla with Separate Bile and Pancreatic Duct Orifices Surinder Singh Rana, Deepak Kumar Bhasin Department of Gastroenterology, Post Graduate Institute of Medical Education and Research (PGIMER). Chandigarh, India A 32-year-old male, a known case of alcohol related Conflict of interest The authors have no potential chronic non calcific pancreatitis, was referred to us for conflicts of interest pancreatic endotherapy for relief of intractable abdominal pain. The cross sectional imaging studies References had revealed an irregularly dilated main pancreatic duct. The examination of the major duodenal papilla 1. Silvis SE, Vennes JA, Dreyer M. Variation in the normal duodenal papilla. Gastrointest Endosc 1983; 29:132-133 [PMID; revealed the presence of two separate orifices at 6852473] endoscopic retrograde cholangiopancreatography (ERCP) (Image). The cranial orifice was located at 11- 12 clock position whereas the caudal orifice was located at 4-5 clock position. The caudal orifice was selectively cannulated and the injection of the contrast revealed presence of an irregularly dilated main pancreatic duct. The cannula and the guide wire introduced through the caudal orifice selectively entered the pancreatic duct and did not come out through the cranial orifice. During ERCP, bile could be seen coming out of the cranial orifice, confirming it to be the orifice of common bile duct. Following selective cannulation of the main pancreatic duct, a 5-Fr stent was placed into the pancreatic duct. Following this, the patient had complete pain relief and is planned for further sessions of pancreatic endotherapy along with pancreatic sphincterotomy. -

4L Eosinophilic Granuloma of Gastro-Intestinal Tract Caused by Herring Parasite Eustoma Rotundatum
BsrrmsH 2 May 1964 MEDICAL JOURNAL 1141 4L Eosinophilic Granuloma of Gastro-intestinal Tract Caused by Herring Parasite Eustoma rotundatum B. STERRY ASHBY,* M.B., F.R.C.S.; P. J. APPLETONt M.B., B.S. IAN DAWSON,4 M.D., M.R.C.P. Brit. med.JY., 1964, 1, 1141-1145 For over 25 years sporadic reports have been appearing in the came from many countries in many different parts of the world. literature of cases of eosinophilic granuloma arising in various The essential details of these cases are recorded in Table I parts of the gastro-intestinal tract. Kaiiser (1937) described and Fig. 1. the first cases. In a search of the literature, which although The one feature common to all these case reports is the extensive is not claimed to be exhaustive, 47 papers were microscopical appearance of the lesion. No matter which part found, describing a total of 89 cases. They occurred through- of the alimentary tract is involved, the histological description out the alimentary tract from pharynx to rectum, though the is the same-an oedematous connective-tissue stroma with an majority were in the stomach and small intestine, and they increase of capillaries and lymphatics, and showing a massive diffuse eosinophil-cell infiltration, usually confined to the sub- * Surgical Registrar, Westminster Hospital and Medical School London. the muscularis mucosae and t House-Surgeon, Westminster Hospital and Medical School, Iondon. mucosa but sometimes splitting t Reader in Pathology, Westminster Hospital and Medical School, spreading into the muscle layer. The mucosa is almost always London. intact. -

Studies of the Function of the Human Pylorus : and Its Role in The
+.1 Studúes OlTlæ Ftrnctíon OJTIrc Humanfolonts And,Iß R.ole InTlæ Riegulø;tíon OÍ Cústríß Drnptging David R. Fone Departments of Medicine and Gastroenterology, Royal Adelaide Hospital University of Adelaide August 1990 Table of Contents TABLE OF CONTENTS . SUMMARY vil DECLARATION...... X DED|CAT|ON.. .. ... xt ACKNOWLEDGMENTS xil CHAPTER 1 ANATOMY OF THE PYLORUS 1.1 INTRODUCTION.. 1 1.2 MUSCULAR ANATOMY 2 1.3 MUCOSAL ANATOMY 4 1.4 NEURALANATOMY 1.4.1 Extrinsic lnnervation of the Pylorus 5 1.4.2 lntrinsic lnnervation of the Pylorus 7 1.5 INTERSTITIAL CELLS OF CAJAL 8 1.6 CONCLUSTON 9 CHAPTER 2 MEASUREMENT OF PYLORIC MOTILITY 2.1 INTRODUCTION 10 2.2 METHODOLOG ICAL CHALLENGES 2.2.1 The Anatomical Mobility of the Pylorus . 10 2.2.2 The Narrowness of the Zone of Pyloric Contraction 12 2.3 METHODS USED TO MEASURE PYLORIC MOTILITY 2.3.1 lntraluminal Techniques 2.3.1.1 Balloon Measurements. 12 t 2.3 1.2 lntraluminal Side-hole Manometry . 13 2.3 1.9 The Sleeve Sensor 14 2.3 1.4 Endoscopy. 16 2.3 1.5 Measurements of Transpyloric Flow . 16 2.3 'I .6 lmpedance Electrodes 16 2.3.2 Extraluminal Techniques For Recording Pyl;'; l'¡"r¡iit¡l 2.3.2.'t Strain Gauges . 17 2.3.2.2 lnduction Coils . 17 2.3.2.3 Electromyography 17 2.3.3 Non-lnvasive Approaches For Recording 2.3.3.1 Radiology :ï:: Y:1":'1 18 2.3.9.2 Ultrasonography . 1B 2.3.3.3 Electrogastrography 19 2.3.4 ln Vitro Studies of Pyloric Muscle 19 2.4 CONCLUSTON. -

GI Motility-I (Deglutition and Motor Functions of Stomach)
Lecture series Gastrointestinal tract Professor Shraddha Singh, Department of Physiology, KGMU, Lucknow GASTROINTESTINAL MOTILITY-1 (Deglutation and motor function of stomach) Mastication (Chewing) - the anterior teeth (incisors) providing a strong cutting action and the posterior teeth (molars), a grinding action – canine tearing - All the jaw muscles working together can close the teeth with a force as great as 25 Kg on the incisors and 90 Kg on the molars. - Most of the muscles of chewing are innervated by the motor branch of the 5th cranial nerve, and the chewing process is controlled by nuclei in the brain stem. - Stimulation of areas in the hypothalamus, amygdala, and even the cerebral cortex near the sensory areas for taste and smell can often cause chewing Chewing Reflex - The presence of a bolus of food in the mouth at first initiates reflex inhibition of the muscles of mastication, which allows the lower jaw to drop. ↓ - The drop in turn initiates a stretch reflex of the jaw muscles that leads to rebound contraction ↓ - raises the jaw to cause closure of the teeth ↓ - compresses the bolus again against the linings of the mouth, which inhibits the jaw muscles once again Chewing Reflex - most fruits and raw vegetables because these have indigestible cellulose membranes around their nutrient portions that must be broken before the food can be digested - Digestive enzymes act only on the surfaces of food particles - grinding the food to a very fine particulate consistency prevents excoriation of the GIT (Stomach) Swallowing (Deglutition) -

A Rare Case of Esophageal Metastasis from Pancreatic Ductal Adenocarcinoma: a Case Report and Literature Review
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 59), pp: 100942-100950 Case Report A rare case of esophageal metastasis from pancreatic ductal adenocarcinoma: a case report and literature review Lauren M. Rosati1,*, Megan N. Kummerlowe1,*, Justin Poling2, Amy Hacker-Prietz1, Amol K. Narang1, Eun J. Shin3, Dung T. Le4, Elliot K. Fishman5, Ralph H. Hruban2, Stephen C. Yang6, Matthew J. Weiss6 and Joseph M. Herman1,7 1 Department of Radiation Oncology & Molecular Radiation Sciences, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Department of Pathology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Department of Gastroenterology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 4 Department of Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 5 Department of Radiology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 6 Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 7 Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA * These authors have contributed equally to this manuscript Correspondence to: Joseph M. Herman, email: [email protected] Keywords: pancreatic cancer, pancreatic ductal adenocarcinoma, metastatic, esophagus, esophageal metastasis Received: April 28, 2017 Accepted: May 20, 2017 Published: June 12, 2017 Copyright: Rosati et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

The Arrest of Intestinal Epithelial ' Turnover' by the Use of X-Irradiation
Gut, 1962, 3, 26 Gut: first published as 10.1136/gut.3.1.26 on 1 March 1962. Downloaded from The arrest of intestinal epithelial ' turnover' by the use of x-irradiation G. WIERNIK, R. G. SHORTER, AND B. CREAMER From St. Thomas's Hospital and Medical School, London sYNoPsIs Mitotic activity and hence cell turnover has been abolished by x-irradiation with 1,000 r. in segments of the rat's gut. The whole stomach, the ascending colon, and 5 cm. of jejunum and ileum were irradiated and the changes observed histologically up to three days. Cessation of cell turnover in the small intestine is followed by cell death and desquamation within 24 hours, leading to denudation of the epithelium and collapse of villous structure. The pylorus and colon showed similar but less marked changes. In contrast the fundus and body of the stomach were unaffected by the absence of mitosis over a period of three days. It is concluded that cell turnover is, in part, necessary to maintain the mucosal structure, particularly the villous pattern in the small intestine. The gastro-intestinal epithelium is a dynamic METHODS structure. Although it appears an intact and lush Wistar rats of either sex weighing 200 g. were used. The membrane to histological examination, it is being animals were anaesthetized with intraperitoneal pento- continuously replaced and desquamated extremely barbitone sodium B.P. The segment of gut to be irradiated quickly. This replacement ofcells results from intense was delivered through an abdominal incision and its http://gut.bmj.com/ division in the crypts, the cells so formed migrating extent marked by fine silk loops. -

The Gallbladder
The Gallbladder Anatomy of the gallbladder Location: Right cranial abdominal quadrant. In the gallbladder fossa of the liver. o Between the quadrate and right medial liver lobes. Macroscopic: Pear-shaped organ Fundus, body and neck. o Neck attaches, via a short cystic duct, to the common bile duct. Opens into the duodenum via sphincter of Oddi at the major duodenal papilla. Found on the mesenteric margin of orad duodenum. o 3-6 cm aboral to pylorus. 1-2cm of distal common bile duct runs intramural. Species differences: Dogs: o Common bile duct enters at major duodenal papilla. Adjacent to pancreatic duct (no confluence prior to entrance). o Accessory pancreatic duct enters at minor duodenal papilla. ± 2 cm aboral to major duodenal papilla. MAJOR conduit for pancreatic secretions. Cats: o Common bile duct and pancreatic duct converge before opening at major duodenal papilla. Thus, any surgical procedure that affects the major duodenal papilla can affect the exocrine pancreatic secretions in cats. o Accessory pancreatic duct only seen in 20% of cats. 1 Gallbladder wall: 5 histologically distinct layers. From innermost these include: o Epithelium, o Submucosa (consisting of the lamina propria and tunica submucosa), o Tunica muscularis externa, o Tunica serosa (outermost layer covers gallbladder facing away from the liver), o Tunica adventitia (outermost layer covers gallbladder facing towards the liver). Blood supply: Solely by the cystic artery (derived from the left branch of the hepatic artery). o Susceptible to ischaemic necrosis should its vascular supply become compromised. Function: Storage reservoir for bile o Concentrated (up to 10-fold), acidified (through epithelial acid secretions) and modified (by the addition of mucin and immunoglobulins) before being released into the gastrointestinal tract at the major duodenal. -

Normal Pancreatic Function 1. What Are the Functions of the Pancreas?
Normal Pancreatic Function Stephen J. Pandol Cedars-Sinai Medical Center and Department of Veterans Affairs Los Angeles, California USA [email protected] Version 1.0, June 13, 2015 [DOI: 10.3998/panc.2015.17] 1. What are the functions of the This chapter presents processes underlying the functions of the exocrine pancreas with pancreas? references to how specific abnormalities of the The pancreas has both exocrine and endocrine pancreas can lead to disease states. function. This chapter is devoted to the exocrine functions of the pancreas. The exocrine function 2. Where is the pancreas located? is devoted to secretion of digestive enzymes, ions and water into the intestine of the gastrointestinal The illustration in Figure 1 demonstrates the (GI) tract. The digestive enzymes are necessary anatomical relationships between the pancreas for converting a meal into molecules that can be and organs surrounding it in the abdomen. The absorbed across the surface lining of the GI tract regions of the pancreas are the head, body, tail into the body. Of note, there are digestive and uncinate process (Figure 2). The distal end enzymes secreted by our salivary glands, of the common bile duct passes through the head stomach and surface epithelium of the GI tract of the pancreas and joins the pancreatic duct as it that also contribute to digestion of a meal. enters the intestine (Figure 2). Because the bile However, the exocrine pancreas is necessary for duct passes through the pancreas before entering most of the digestion of a meal and without it the intestine, diseases of the pancreas such as a there is a substantial loss of digestion that results cancer at the head of the pancreas or swelling in malnutrition. -

Effect of Chronic Ethanol Ingestion on Enterocyte Turnover in Rat Small Intestine
Gut: first published as 10.1136/gut.28.1.52 on 1 January 1987. Downloaded from Gut, 1987, 28, 52-55 Effect of chronic ethanol ingestion on enterocyte turnover in rat small intestine R MAZZANTI AND W J JENKINS From the Department of' Medicine, Royal Free Hospital School of Medicine, Rowland Hill Street, Londlon SUMMARY Whether chronic ethanol ingestion significantly damages the small intestine remains controversial. To clarify this we have analysed the morphology of the small intestinal epithelium and quantified its renewal in chronically ethanol fed rats. Twenty adult male rats were pair fed for 28 days a nutritionally adequate liquid diet containing either ethanol as 36% of total calories or an isocaloric diet in which fat substituted for ethanol. Crypt cell production rate was determined in the jejunum and ileum by the metaphase arrest method. Weight gain and small intestinal morphology were similar in ethanol fed and control rats, but enterocyte turnover was significantly reduced in the jejunum (p<O.05) and ileum (p<0.0l) of the ethanol fed rats. This effect of ethanol on the small intestine is probably systemic rather than local, because the changes in jejunum and ileum were similar, and it may contribute to the development of malnutrition in chronic alcoholics. A variety of changes in intestinal function have Methods been reported after acute or habitual alcohol consumption.' Impaired absorption of vitamins,25 ANIMALS sugars6 7 and amino acids8 9 has been shown in Twenty adult male Sprague-Dawley rats, which http://gut.bmj.com/ the presence of ethanol, and the chronic consump- initially weighed between 210 g and 310 g, were tion of ethanol is often associated with malnutrition maintained in single cages on a 12 hour light-dark and diarrhoea, but whether these are caused by cycle at 21 ±1°C room temperature. -

Pancreaticobiliary Ductal Union Gut: First Published As 10.1136/Gut.31.10.1144 on 1 October 1990
1144 Gut, 1990, 31, 1144-1149 Pancreaticobiliary ductal union Gut: first published as 10.1136/gut.31.10.1144 on 1 October 1990. Downloaded from S P Misra, M Dwivedi Abstract TABLE ii Length ofthe common channel in normalsubjects in The main pancreatic duct and the common bile various series duct open into the second part of the duo- Authors Mean (mm) Range (mm) denum alone or after joining as a common Misra et al4 4-7 1-018-4 channel. A common channel of >15 mm (an Kimura et al5 4.6 2-10 anomalous pancreaticobiliary duct) is associ- Dowdy et al6 4-4 1-12 ated with congenital cystic dilatation of the common bile duct and carcinoma of the gall bladder. Even a long common channel channel of <3 mm, and 18% had a common (38 mm) is associated with a higher frequency channel of >3 mm. The rhean length was not of carcinoma of the gall bladder. Gail stones mentioned.2 In a necropsy study of 35 infants, smaller than the common channel and a long Miyano et al' noted that the average length of the common channel predispose to gail stone common channel was 1 3 mm. induced acute pancreatitis. Separate openings Kimura et al,' using cineradiography during for the two ductal systems predisposes to ERCP, have shown contractile motility of the development of gall stones and alcohol ductal wall extending well beyond the common induced chronic pancreatitis. The role of channel, towards the liver. The mean (SD) ductal union has also been investigated in length of the contractile segment was 20 5 primary sclerosing cholangitis and biliary (4 6) mm (range 14-31 mm). -
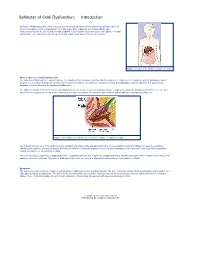
Sphincter of Oddi Dysfunction: Introduction
Sphincter of Oddi Dysfunction: Introduction Sphincter of Oddi dysfunction refers to structural or functional disorders involving the biliary sphincter that may result in impedance of bile and pancreatic juice flow. Up to 20% of patients with continued pain after cholecystectomy and 10–20% of patients with idiopathic recurrent pancreatitis may suffer from sphincter of Oddi dysfunction. This condition is more prevalent among middle-aged women for unclear reasons Figure 1. Location of the sphincter of Oddi in the body. What is Sphincter of Oddi Dysfunction? The sphincter of Oddi has three major functions: 1) regulation of bile and pancreatic flow into the duodenum, 2) diversion of hepatic bile into the gallbladder, and 3) the prevention of reflux of duodenal contents into the pancreaticobiliary tract. With the ingestion of a meal, the gallbladder contracts and there is a simultaneous decrease in the resistance in the sphincter of Oddi zone. The sphincter of Oddi consists of circular and longitudinal smooth muscle fibers surrounding a variable length of the distal bile and pancreatic duct. There are three discrete areas of muscle thickness, or mini sphincters: the sphincter papillae, the sphincter pancreaticus, and the sphincter choledochus (Figure 2). Figure 2. Mini sphincters, or discrete areas of muscle, comprise the sphincter of Oddi. The major physiologic role of the sphincter is the regulation of the flow of bile and pancreatic juice. Cholecystokinin (CCK) and nitrates decrease the resistance offered by the sphincter. Laboratory studies observing the effects of numerous peptides, hormones, and medications on the sphincter have suggested a multifactor control mechanism for the sphincter of Oddi. -

Chronic Pancreatitis: Introduction
Chronic Pancreatitis: Introduction Authors: Anthony N. Kalloo, MD; Lynn Norwitz, BS; Charles J. Yeo, MD Chronic pancreatitis is a relatively rare disorder occurring in about 20 per 100,000 population. The disease is progressive with persistent inflammation leading to damage and/or destruction of the pancreas . Endocrine and exocrine functional impairment results from the irreversible pancreatic injury. The pancreas is located deep in the retroperitoneal space of the upper part of the abdomen (Figure 1). It is almost completely covered by the stomach and duodenum . This elongated gland (12–20 cm in the adult) has a lobe-like structure. Variation in shape and exact body location is common. In most people, the larger part of the gland's head is located to the right of the spine or directly over the spinal column and extends to the spleen . The pancreas has both exocrine and endocrine functions. In its exocrine capacity, the acinar cells produce digestive juices, which are secreted into the intestine and are essential in the breakdown and metabolism of proteins, fats and carbohydrates. In its endocrine function capacity, the pancreas also produces insulin and glucagon , which are secreted into the blood to regulate glucose levels. Figure 1. Location of the pancreas in the body. What is Chronic Pancreatitis? Chronic pancreatitis is characterized by inflammatory changes of the pancreas involving some or all of the following: fibrosis, calcification, pancreatic ductal inflammation, and pancreatic stone formation (Figure 2). Although autopsies indicate that there is a 0.5–5% incidence of pancreatitis, the true prevalence is unknown. In recent years, there have been several attempts to classify chronic pancreatitis, but these have met with difficulty for several reasons.