Stem Cells Versus Plasticity in Liver and Pancreas Regeneration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papilla with Separate Bile and Pancreatic Duct Orifices
JOP. J Pancreas (Online) 2013 May 10; 14(3):302-303. MULTIMEDIA ARTICLE – Clinical Imaging Papilla with Separate Bile and Pancreatic Duct Orifices Surinder Singh Rana, Deepak Kumar Bhasin Department of Gastroenterology, Post Graduate Institute of Medical Education and Research (PGIMER). Chandigarh, India A 32-year-old male, a known case of alcohol related Conflict of interest The authors have no potential chronic non calcific pancreatitis, was referred to us for conflicts of interest pancreatic endotherapy for relief of intractable abdominal pain. The cross sectional imaging studies References had revealed an irregularly dilated main pancreatic duct. The examination of the major duodenal papilla 1. Silvis SE, Vennes JA, Dreyer M. Variation in the normal duodenal papilla. Gastrointest Endosc 1983; 29:132-133 [PMID; revealed the presence of two separate orifices at 6852473] endoscopic retrograde cholangiopancreatography (ERCP) (Image). The cranial orifice was located at 11- 12 clock position whereas the caudal orifice was located at 4-5 clock position. The caudal orifice was selectively cannulated and the injection of the contrast revealed presence of an irregularly dilated main pancreatic duct. The cannula and the guide wire introduced through the caudal orifice selectively entered the pancreatic duct and did not come out through the cranial orifice. During ERCP, bile could be seen coming out of the cranial orifice, confirming it to be the orifice of common bile duct. Following selective cannulation of the main pancreatic duct, a 5-Fr stent was placed into the pancreatic duct. Following this, the patient had complete pain relief and is planned for further sessions of pancreatic endotherapy along with pancreatic sphincterotomy. -

A Rare Case of Esophageal Metastasis from Pancreatic Ductal Adenocarcinoma: a Case Report and Literature Review
www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 59), pp: 100942-100950 Case Report A rare case of esophageal metastasis from pancreatic ductal adenocarcinoma: a case report and literature review Lauren M. Rosati1,*, Megan N. Kummerlowe1,*, Justin Poling2, Amy Hacker-Prietz1, Amol K. Narang1, Eun J. Shin3, Dung T. Le4, Elliot K. Fishman5, Ralph H. Hruban2, Stephen C. Yang6, Matthew J. Weiss6 and Joseph M. Herman1,7 1 Department of Radiation Oncology & Molecular Radiation Sciences, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 2 Department of Pathology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 3 Department of Gastroenterology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 4 Department of Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 5 Department of Radiology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 6 Department of Surgery, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins University School of Medicine, Baltimore, MD, USA 7 Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA * These authors have contributed equally to this manuscript Correspondence to: Joseph M. Herman, email: [email protected] Keywords: pancreatic cancer, pancreatic ductal adenocarcinoma, metastatic, esophagus, esophageal metastasis Received: April 28, 2017 Accepted: May 20, 2017 Published: June 12, 2017 Copyright: Rosati et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Normal Pancreatic Function 1. What Are the Functions of the Pancreas?
Normal Pancreatic Function Stephen J. Pandol Cedars-Sinai Medical Center and Department of Veterans Affairs Los Angeles, California USA [email protected] Version 1.0, June 13, 2015 [DOI: 10.3998/panc.2015.17] 1. What are the functions of the This chapter presents processes underlying the functions of the exocrine pancreas with pancreas? references to how specific abnormalities of the The pancreas has both exocrine and endocrine pancreas can lead to disease states. function. This chapter is devoted to the exocrine functions of the pancreas. The exocrine function 2. Where is the pancreas located? is devoted to secretion of digestive enzymes, ions and water into the intestine of the gastrointestinal The illustration in Figure 1 demonstrates the (GI) tract. The digestive enzymes are necessary anatomical relationships between the pancreas for converting a meal into molecules that can be and organs surrounding it in the abdomen. The absorbed across the surface lining of the GI tract regions of the pancreas are the head, body, tail into the body. Of note, there are digestive and uncinate process (Figure 2). The distal end enzymes secreted by our salivary glands, of the common bile duct passes through the head stomach and surface epithelium of the GI tract of the pancreas and joins the pancreatic duct as it that also contribute to digestion of a meal. enters the intestine (Figure 2). Because the bile However, the exocrine pancreas is necessary for duct passes through the pancreas before entering most of the digestion of a meal and without it the intestine, diseases of the pancreas such as a there is a substantial loss of digestion that results cancer at the head of the pancreas or swelling in malnutrition. -

Pancreaticobiliary Ductal Union Gut: First Published As 10.1136/Gut.31.10.1144 on 1 October 1990
1144 Gut, 1990, 31, 1144-1149 Pancreaticobiliary ductal union Gut: first published as 10.1136/gut.31.10.1144 on 1 October 1990. Downloaded from S P Misra, M Dwivedi Abstract TABLE ii Length ofthe common channel in normalsubjects in The main pancreatic duct and the common bile various series duct open into the second part of the duo- Authors Mean (mm) Range (mm) denum alone or after joining as a common Misra et al4 4-7 1-018-4 channel. A common channel of >15 mm (an Kimura et al5 4.6 2-10 anomalous pancreaticobiliary duct) is associ- Dowdy et al6 4-4 1-12 ated with congenital cystic dilatation of the common bile duct and carcinoma of the gall bladder. Even a long common channel channel of <3 mm, and 18% had a common (38 mm) is associated with a higher frequency channel of >3 mm. The rhean length was not of carcinoma of the gall bladder. Gail stones mentioned.2 In a necropsy study of 35 infants, smaller than the common channel and a long Miyano et al' noted that the average length of the common channel predispose to gail stone common channel was 1 3 mm. induced acute pancreatitis. Separate openings Kimura et al,' using cineradiography during for the two ductal systems predisposes to ERCP, have shown contractile motility of the development of gall stones and alcohol ductal wall extending well beyond the common induced chronic pancreatitis. The role of channel, towards the liver. The mean (SD) ductal union has also been investigated in length of the contractile segment was 20 5 primary sclerosing cholangitis and biliary (4 6) mm (range 14-31 mm). -
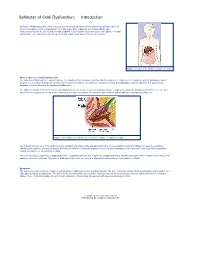
Sphincter of Oddi Dysfunction: Introduction
Sphincter of Oddi Dysfunction: Introduction Sphincter of Oddi dysfunction refers to structural or functional disorders involving the biliary sphincter that may result in impedance of bile and pancreatic juice flow. Up to 20% of patients with continued pain after cholecystectomy and 10–20% of patients with idiopathic recurrent pancreatitis may suffer from sphincter of Oddi dysfunction. This condition is more prevalent among middle-aged women for unclear reasons Figure 1. Location of the sphincter of Oddi in the body. What is Sphincter of Oddi Dysfunction? The sphincter of Oddi has three major functions: 1) regulation of bile and pancreatic flow into the duodenum, 2) diversion of hepatic bile into the gallbladder, and 3) the prevention of reflux of duodenal contents into the pancreaticobiliary tract. With the ingestion of a meal, the gallbladder contracts and there is a simultaneous decrease in the resistance in the sphincter of Oddi zone. The sphincter of Oddi consists of circular and longitudinal smooth muscle fibers surrounding a variable length of the distal bile and pancreatic duct. There are three discrete areas of muscle thickness, or mini sphincters: the sphincter papillae, the sphincter pancreaticus, and the sphincter choledochus (Figure 2). Figure 2. Mini sphincters, or discrete areas of muscle, comprise the sphincter of Oddi. The major physiologic role of the sphincter is the regulation of the flow of bile and pancreatic juice. Cholecystokinin (CCK) and nitrates decrease the resistance offered by the sphincter. Laboratory studies observing the effects of numerous peptides, hormones, and medications on the sphincter have suggested a multifactor control mechanism for the sphincter of Oddi. -

Chronic Pancreatitis: Introduction
Chronic Pancreatitis: Introduction Authors: Anthony N. Kalloo, MD; Lynn Norwitz, BS; Charles J. Yeo, MD Chronic pancreatitis is a relatively rare disorder occurring in about 20 per 100,000 population. The disease is progressive with persistent inflammation leading to damage and/or destruction of the pancreas . Endocrine and exocrine functional impairment results from the irreversible pancreatic injury. The pancreas is located deep in the retroperitoneal space of the upper part of the abdomen (Figure 1). It is almost completely covered by the stomach and duodenum . This elongated gland (12–20 cm in the adult) has a lobe-like structure. Variation in shape and exact body location is common. In most people, the larger part of the gland's head is located to the right of the spine or directly over the spinal column and extends to the spleen . The pancreas has both exocrine and endocrine functions. In its exocrine capacity, the acinar cells produce digestive juices, which are secreted into the intestine and are essential in the breakdown and metabolism of proteins, fats and carbohydrates. In its endocrine function capacity, the pancreas also produces insulin and glucagon , which are secreted into the blood to regulate glucose levels. Figure 1. Location of the pancreas in the body. What is Chronic Pancreatitis? Chronic pancreatitis is characterized by inflammatory changes of the pancreas involving some or all of the following: fibrosis, calcification, pancreatic ductal inflammation, and pancreatic stone formation (Figure 2). Although autopsies indicate that there is a 0.5–5% incidence of pancreatitis, the true prevalence is unknown. In recent years, there have been several attempts to classify chronic pancreatitis, but these have met with difficulty for several reasons. -

Pancreas & Biliary System
Pancreas & Biliary System Gastrointestinal block-Anatomy-Lecture 4 Editing file Objectives Color guide : Only in boys slides in Green Only in girls slides in Purple important in Red At the end of the lecture, students should be able to: Notes in Grey ● Describe the location, surface anatomy, parts, relations & peritoneal reflection of the pancreas and gallbladder. ● Describe blood supply, nerve supply andlymphatic drainage of pancreas and gallbladder. ● Describe Course of each of common hepatic, cystic and common bile duct and pancreatic ducts Pancreas Location ● Located in Epigastrium & Left upper quadrant (left hypochondriac) of abdomen behind the stomach. in front of spleen (from concavity of the duodenum to the hilum of spleen opposite the level of T12– L3 vertebrae). ● 12–15 cm ,6-10 inch in length and 60-100 gram in weight. ● soft, lobulated elongated gland ● The greater part is Retroperitoneal behind the lesser sac. ● “J”-shaped or RETORT shaped ● Lies across the posterior abdominal wall in a transverse/oblique directions at the transpyloric plane (L1 vertebra) (except the tail it lies at the level of T12) has exocrine and endocrine functions. Endocrine component Exocrine component ● makes and secretes hormones (insulin, ● makes and secretes digestive glucagon, somatostatin) enzymes into the intestine ● control energy metabolism and storage (Exocrine pancreas) throughout the body (Endocrine ● comprise more than 95% of the pancreas Islet's of Langerhans). pancreatic mass ● comprise 1-2% of pancreatic mass 3 Pancreas Parts 1 Head 2 Neck 3 Body 4 Tail Head ● enlarged, disc-shaped right end of the pancreas ● lies in the concavity of the C-shaped duodenal loop in front of the L2 vertebra. -

Ta2, Part Iii
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART III Contents: Systemata visceralia Visceral systems Caput V: Systema digestorium Chapter 5: Digestive system Caput VI: Systema respiratorium Chapter 6: Respiratory system Caput VII: Cavitas thoracis Chapter 7: Thoracic cavity Caput VIII: Systema urinarium Chapter 8: Urinary system Caput IX: Systemata genitalia Chapter 9: Genital systems Caput X: Cavitas abdominopelvica Chapter 10: Abdominopelvic cavity Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: SYSTEMA DIGESTORIUM Chapter 5: DIGESTIVE SYSTEM Latin term Latin synonym UK English US English English synonym Other 2772 Systemata visceralia Visceral systems Visceral systems Splanchnologia 2773 Systema digestorium Systema alimentarium Digestive system Digestive system Alimentary system Apparatus digestorius; Gastrointestinal system 2774 Stoma Ostium orale; Os Mouth Mouth 2775 Labia oris Lips Lips See Anatomia generalis (Ch. -

The Digestive System
THE DIGESTIVE SYSTEM COMPILED BY HOWIE BAUM DIGESTIVE SYSTEM People are probably more aware of their digestive system than of any other system, not least because of its frequent messages. Hunger, thirst, appetite, gas ☺, and the frequency and nature of bowel movements, are all issues affecting daily life. The Digestive Tract • Six Functions of the Digestive System 1. Ingestion 2. Mechanical processing 3. Digestion 4. Secretion 5. Absorption 6. Excretion The Digestive Tract • Ingestion – Occurs when materials enter digestive tract via the mouth • Mechanical Processing – Crushing and shearing – Makes materials easier to propel along digestive tract • Digestion – The chemical breakdown of food into small organic fragments for absorption by digestive epithelium The Digestive Tract • Secretion – Is the release of water, acids, enzymes, buffers, and salts – By epithelium of digestive tract – By glandular organs • Absorption – Movement of organic substrates, electrolytes, vitamins, and water – Across digestive epithelium tissue – Into the interstitial fluid of digestive tract • Excretion – Removal of waste products from body fluids – Process called defecation removes feces AN INTRODUCTION TO THE DIGESTIVE SYSTEM • The Digestive Tract • Also called the gastrointestinal (GI) tract or alimentary canal • Is a muscular tube • Extends from our mouth to the anus • Passes through the pharynx, esophagus, stomach, and small and large intestines The digestive system is one of the most clearly defined in the body. It consists of a long passageway, the digestive -

Pancreatic Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis
cancer.org | 1.800.227.2345 Pancreatic Cancer Early Detection, Diagnosis, and Staging Detection and Diagnosis Catching cancer early often allows for more treatment options. Some early cancers may have signs and symptoms that can be noticed, but that is not always the case. ● Can Pancreatic Cancer Be Found Early? ● Signs and Symptoms of Pancreatic Cancer ● Tests for Pancreatic Cancer Stages and Outlook (Prognosis) After a cancer diagnosis, staging provides important information about the extent of cancer in the body and anticipated response to treatment. ● Pancreatic Cancer Stages ● Survival Rates for Pancreatic Cancer Questions to Ask About Pancreatic Cancer Here are some questions you can ask your cancer care team to help you better understand your cancer diagnosis and treatment options. ● Questions to Ask About Pancreatic Cancer 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Can Pancreatic Cancer Be Found Early? Pancreatic cancer is hard to find early. The pancreas is deep inside the body, so early tumors can’t be seen or felt by health care providers during routine physical exams. People usually have no symptoms until the cancer has become very large or has already spread to other organs. For certain types of cancer, screening tests or exams are used to look for cancer in people who have no symptoms (and who have not had that cancer before). But for pancreatic cancer, no major professional groups currently recommend routine screening in people who are at average risk. This is because no screening test has been shown to lower the risk of dying from this cancer. Genetic testing for people who might be at increased risk Some people might be at increased risk of pancreatic cancer because of a family history of the disease (or a family history of certain other cancers). -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

Axis Scientific Human Digestive System (1/2 Size)
Axis Scientific Human Digestive System (1/2 Size) A-105865 48. Body of Pancreas 27. Transverse Colon 02. Hard Palate 47. Pancreatic Notch 07. Nasopharynx 05. Tooth 01. Lower Lip F. Large Intestine 06. Tongue 21. Jejunum A. Oral Cavity 09. Pharyngeal Tonsil 03. Soft Palate 08. Opening to Auditory Tube 28. E. Small Intestine 04. Uvula 11. Palatine Tonsil Descending Colon 40. Gallbladder 37. Round Ligament of Liver 22. Ileum 38. Quadrate Lobe 44. Proper Hepatic Artery 42. Common Hepatic Duct 45. Hepatic Portal Vein C. Esophagus 15. Fundus of Stomach 30. Rectum 29. Sigmoid Colon 13. Cardia 26. Ascending Colon 39. Caudate Lobe 24. Ileocecal Valve 35. Left Lobe of 16. Body of 34. Falciform Liver Stomach 41. Cystic Duct Ligament 36. Right Lobe of Liver G. Liver 31. Anal Canal 14. Pylorus D. Stomach 33. External 18. Duodenum Anal Sphincter Muscle 17. Pyloric Antrum 46. Head of Pancreas 23. Cecum 51. Accessory Pancreatic Duct 49. Tail of 50. Pancreatic 25. Vermiform 20. Minor Duodenal Papilla H. Pancreas Duct Pancreas Appendix 19. Major Duodenal Papilla 32. Internal Anal Sphincter Muscle 01. Lower Lip 20. Minor Duodenal Papilla 39. Caudate Lobe 02. Hard Palate 21. Jejunum 40. Gallbladder 03. Soft Palate 22. Ileum 41. Cystic Duct 42. Common Hepatic Duct 04. Uvula 23. Cecum 43. Common Bile Duct 05. Tooth 24. Ileocecal Valve 44. Proper Hepatic Artery 06. Tongue 25. Vermiform Appendix 45. Hepatic Portal Vein 07. Nasopharynx 26. Ascending Colon 46. Head of Pancreas 08. Opening to Auditory Tube 27. Transverse Colon 47. Pancreatic Notch 09. Pharyngeal Tonsil 28.