Three-Dimensionally Preserved Minute Larva of a Great-Appendage Arthropod from the Early Cambrian Chengjiang Biota
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibious Fishes: Terrestrial Locomotion, Performance, Orientation, and Behaviors from an Applied Perspective by Noah R
AMPHIBIOUS FISHES: TERRESTRIAL LOCOMOTION, PERFORMANCE, ORIENTATION, AND BEHAVIORS FROM AN APPLIED PERSPECTIVE BY NOAH R. BRESSMAN A Dissertation Submitted to the Graduate Faculty of WAKE FOREST UNIVESITY GRADUATE SCHOOL OF ARTS AND SCIENCES in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Biology May 2020 Winston-Salem, North Carolina Approved By: Miriam A. Ashley-Ross, Ph.D., Advisor Alice C. Gibb, Ph.D., Chair T. Michael Anderson, Ph.D. Bill Conner, Ph.D. Glen Mars, Ph.D. ACKNOWLEDGEMENTS I would like to thank my adviser Dr. Miriam Ashley-Ross for mentoring me and providing all of her support throughout my doctoral program. I would also like to thank the rest of my committee – Drs. T. Michael Anderson, Glen Marrs, Alice Gibb, and Bill Conner – for teaching me new skills and supporting me along the way. My dissertation research would not have been possible without the help of my collaborators, Drs. Jeff Hill, Joe Love, and Ben Perlman. Additionally, I am very appreciative of the many undergraduate and high school students who helped me collect and analyze data – Mark Simms, Tyler King, Caroline Horne, John Crumpler, John S. Gallen, Emily Lovern, Samir Lalani, Rob Sheppard, Cal Morrison, Imoh Udoh, Harrison McCamy, Laura Miron, and Amaya Pitts. I would like to thank my fellow graduate student labmates – Francesca Giammona, Dan O’Donnell, MC Regan, and Christine Vega – for their support and helping me flesh out ideas. I am appreciative of Dr. Ryan Earley, Dr. Bruce Turner, Allison Durland Donahou, Mary Groves, Tim Groves, Maryland Department of Natural Resources, UF Tropical Aquaculture Lab for providing fish, animal care, and lab space throughout my doctoral research. -
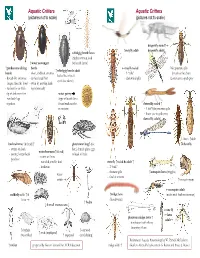
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Evolution of Pycnogonid Life History Traits Eric Carl Lovely University of New Hampshire, Durham
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Winter 1999 Evolution of pycnogonid life history traits Eric Carl Lovely University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Lovely, Eric Carl, "Evolution of pycnogonid life history traits" (1999). Doctoral Dissertations. 1975. https://scholars.unh.edu/dissertation/1975 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

Morphology and Function in the Cambrian Burgess Shale
Haug et al. BMC Evolutionary Biology 2012, 12:162 http://www.biomedcentral.com/1471-2148/12/162 RESEARCH ARTICLE Open Access Morphology and function in the Cambrian Burgess Shale megacheiran arthropod Leanchoilia superlata and the application of a descriptive matrix Joachim T Haug1*, Derek EG Briggs2,3 and Carolin Haug1 Abstract Background: Leanchoilia superlata is one of the best known arthropods from the middle Cambrian Burgess Shale of British Columbia. Here we re-describe the morphology of L. superlata and discuss its possible autecology. The re-description follows a standardized scheme, the descriptive matrix approach, designed to provide a template for descriptions of other megacheiran species. Results: Our findings differ in several respects from previous interpretations. Examples include a more slender body; a possible hypostome; a small specialised second appendage, bringing the number of pairs of head appendages to four; a further sub-division of the great appendage, making it more similar to that of other megacheirans; and a complex joint of the exopod reflecting the arthropod’s swimming capabilities. Conclusions: Different aspects of the morphology, for example, the morphology of the great appendage and the presence of a basipod with strong median armature on the biramous appendages indicate that L. superlata was an active and agile necto-benthic predator (not a scavenger or deposit feeder as previously interpreted). Keywords: Megacheira, Great-appendage arthropods, Chelicerata sensu lato, Descriptive matrix, Active predator Background shared with other species. As a consequence, morpho- The description of species is fundamental to the science logical details in many phylogenetic matrices have to be of zoology, including taxonomy, phylogenetic systema- (re-)interpreted, often without the benefit of a compre- tics, functional morphology and ultimately evolutionary hensive description. -

The Origin and Evolution of Arthropods Graham E
INSIGHT REVIEW NATURE|Vol 457|12 February 2009|doi:10.1038/nature07890 The origin and evolution of arthropods Graham E. Budd1 & Maximilian J. Telford2 The past two decades have witnessed profound changes in our understanding of the evolution of arthropods. Many of these insights derive from the adoption of molecular methods by systematists and developmental biologists, prompting a radical reordering of the relationships among extant arthropod classes and their closest non-arthropod relatives, and shedding light on the developmental basis for the origins of key characteristics. A complementary source of data is the discovery of fossils from several spectacular Cambrian faunas. These fossils form well-characterized groupings, making the broad pattern of Cambrian arthropod systematics increasingly consensual. The arthropods are one of the most familiar and ubiquitous of all ani- Arthropods are monophyletic mal groups. They have far more species than any other phylum, yet Arthropods encompass a great diversity of animal taxa known from the living species are merely the surviving branches of a much greater the Cambrian to the present day. The four living groups — myriapods, diversity of extinct forms. One group of crustacean arthropods, the chelicerates, insects and crustaceans — are known collectively as the barnacles, was studied extensively by Charles Darwin. But the origins Euarthropoda. They are united by a set of distinctive features, most and the evolution of arthropods in general, embedded in what is now notably the clear segmentation of their bodies, a sclerotized cuticle and known as the Cambrian explosion, were a source of considerable con- jointed appendages. Even so, their great diversity has led to consider- cern to him, and he devoted a substantial and anxious section of On able debate over whether they had single (monophyletic) or multiple the Origin of Species1 to discussing this subject: “For instance, I cannot (polyphyletic) origins from a soft-bodied, legless ancestor. -

Evolution of Direct-Developing Larvae: Selection Vs Loss Margaret Snoke Smith,1 Kirk S
Hypotheses Evolution of direct-developing larvae: selection vs loss Margaret Snoke Smith,1 Kirk S. Zigler,2 and Rudolf A. Raff1,3* Summary echinoderms, sea urchins, reveal that a majority of sea urchin Observations of a sea urchin larvae show that most species exhibit one of two life history strategies (Fig. 1).(10) species adopt one of two life history strategies. One strategy is to make numerous small eggs, which develop One strategy is to make many small eggs that develop into a into a larva with a required feeding period in the water larva that must feed and grow for weeks or months in the water column before metamorphosis. In contrast, the second column before metamorphosis. In the water column, these strategy is to make fewer large eggs with a larva that does larvae are dependent on plankton abundance for food and are not feed, which reduces the time to metamorphosis and subject to high levels of predation.(11) The other strategy thus the time spent in the water column. The larvae associated with each strategy have distinct morpholo- involves increasing egg size and maternal provisioning, which gies and developmental processes that reflect their decreases the reliance on planktonic food sources and the feeding requirements, so that those that feed exhibit time to metamorphosis, thus minimizing larval mortality. indirect development with a complex larva, and those that However, assuming finite resources for egg production, do not feed form a morphologically simplified larva and increasing egg size also reduces the total number of eggs exhibit direct development. Phylogenetic studies show that, in sea urchins, a feeding larva, the pluteus, is the produced. -

Arthropoda: Pycnogonida)
European Journal of Taxonomy 286: 1–33 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2017.286 www.europeanjournaloftaxonomy.eu 2017 · Sabroux R. et al. This work is licensed under a Creative Commons Attribution 3.0 License. DNA Library of Life, research article urn:lsid:zoobank.org:pub:8B9DADD0-415E-4120-A10E-8A3411C1C1A4 Biodiversity and phylogeny of Ammotheidae (Arthropoda: Pycnogonida) Romain SABROUX 1, Laure CORBARI 2, Franz KRAPP 3, Céline BONILLO 4, Stépahnie LE PRIEUR 5 & Alexandre HASSANIN 6,* 1,2,6 UMR 7205, Institut de Systématique, Evolution et Biodiversité, Département Systématique et Evolution, Sorbonne Universités, Muséum national d’Histoire naturelle, 55 rue Buffon, CP 51, 75005 Paris, France. 3 Zoologisches Forschungsmuseum Alexander Koenig, Adenauerallee 160, 53113 Bonn, Germany. 4,5 UMS CNRS 2700, Muséum national d’Histoire naturelle, CP 26, 57 rue Cuvier, 75231 Paris Cedex 05, France. * Corresponding author: [email protected] 1 Email: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 1 urn:lsid:zoobank.org:author:F48B4ABE-06BD-41B1-B856-A12BE97F9653 2 urn:lsid:zoobank.org:author:9E5EBA7B-C2F2-4F30-9FD5-1A0E49924F13 3 urn:lsid:zoobank.org:author:331AD231-A810-42F9-AF8A-DDC319AA351A 4 urn:lsid:zoobank.org:author:7333D242-0714-41D7-B2DB-6804F8064B13 5 urn:lsid:zoobank.org:author:5C9F4E71-9D73-459F-BABA-7495853B1981 6 urn:lsid:zoobank.org:author:0DCC3E08-B2BA-4A2C-ADA5-1A256F24DAA1 Abstract. The family Ammotheidae is the most diversified group of the class Pycnogonida, with 297 species described in 20 genera. -

Hookworm-Related Cutaneous Larva Migrans
326 Hookworm-Related Cutaneous Larva Migrans Patrick Hochedez , MD , and Eric Caumes , MD Département des Maladies Infectieuses et Tropicales, Hôpital Pitié-Salpêtrière, Paris, France DOI: 10.1111/j.1708-8305.2007.00148.x Downloaded from https://academic.oup.com/jtm/article/14/5/326/1808671 by guest on 27 September 2021 utaneous larva migrans (CLM) is the most fre- Risk factors for developing HrCLM have specifi - Cquent travel-associated skin disease of tropical cally been investigated in one outbreak in Canadian origin. 1,2 This dermatosis fi rst described as CLM by tourists: less frequent use of protective footwear Lee in 1874 was later attributed to the subcutane- while walking on the beach was signifi cantly associ- ous migration of Ancylostoma larvae by White and ated with a higher risk of developing the disease, Dove in 1929. 3,4 Since then, this skin disease has also with a risk ratio of 4. Moreover, affected patients been called creeping eruption, creeping verminous were somewhat younger than unaffected travelers dermatitis, sand worm eruption, or plumber ’ s itch, (36.9 vs 41.2 yr, p = 0.014). There was no correla- which adds to the confusion. It has been suggested tion between the reported amount of time spent on to name this disease hookworm-related cutaneous the beach and the risk of developing CLM. Consid- larva migrans (HrCLM).5 ering animals in the neighborhood, 90% of the Although frequent, this tropical dermatosis is travelers in that study reported seeing cats on the not suffi ciently well known by Western physicians, beach and around the hotel area, and only 1.5% and this can delay diagnosis and effective treatment. -

Evolutionary Developmental Biology 573
EVOC20 29/08/2003 11:15 AM Page 572 Evolutionary 20 Developmental Biology volutionary developmental biology, now often known Eas “evo-devo,” is the study of the relation between evolution and development. The relation between evolution and development has been the subject of research for many years, and the chapter begins by looking at some classic ideas. However, the subject has been transformed in recent years as the genes that control development have begun to be identified. This chapter looks at how changes in these developmental genes, such as changes in their spatial or temporal expression in the embryo, are associated with changes in adult morphology. The origin of a set of genes controlling development may have opened up new and more flexible ways in which evolution could occur: life may have become more “evolvable.” EVOC20 29/08/2003 11:15 AM Page 573 CHAPTER 20 / Evolutionary Developmental Biology 573 20.1 Changes in development, and the genes controlling development, underlie morphological evolution Morphological structures, such as heads, legs, and tails, are produced in each individual organism by development. The organism begins life as a single cell. The organism grows by cell division, and the various cell types (bone cells, skin cells, and so on) are produced by differentiation within dividing cell lines. When one species evolves into Morphological evolution is driven another, with a changed morphological form, the developmental process must have by developmental evolution changed too. If the descendant species has longer legs, it is because the developmental process that produces legs has been accelerated, or extended over time. -

Segmentation and Tagmosis in Chelicerata
Arthropod Structure & Development 46 (2017) 395e418 Contents lists available at ScienceDirect Arthropod Structure & Development journal homepage: www.elsevier.com/locate/asd Segmentation and tagmosis in Chelicerata * Jason A. Dunlop a, , James C. Lamsdell b a Museum für Naturkunde, Leibniz Institute for Evolution and Biodiversity Science, Invalidenstrasse 43, D-10115 Berlin, Germany b American Museum of Natural History, Division of Paleontology, Central Park West at 79th St, New York, NY 10024, USA article info abstract Article history: Patterns of segmentation and tagmosis are reviewed for Chelicerata. Depending on the outgroup, che- Received 4 April 2016 licerate origins are either among taxa with an anterior tagma of six somites, or taxa in which the ap- Accepted 18 May 2016 pendages of somite I became increasingly raptorial. All Chelicerata have appendage I as a chelate or Available online 21 June 2016 clasp-knife chelicera. The basic trend has obviously been to consolidate food-gathering and walking limbs as a prosoma and respiratory appendages on the opisthosoma. However, the boundary of the Keywords: prosoma is debatable in that some taxa have functionally incorporated somite VII and/or its appendages Arthropoda into the prosoma. Euchelicerata can be defined on having plate-like opisthosomal appendages, further Chelicerata fi Tagmosis modi ed within Arachnida. Total somite counts for Chelicerata range from a maximum of nineteen in Prosoma groups like Scorpiones and the extinct Eurypterida down to seven in modern Pycnogonida. Mites may Opisthosoma also show reduced somite counts, but reconstructing segmentation in these animals remains chal- lenging. Several innovations relating to tagmosis or the appendages borne on particular somites are summarised here as putative apomorphies of individual higher taxa. -

Cooption of an Appendage-Patterning Gene Cassette in the Head
Cooption of an appendage-patterning gene cassette in PNAS PLUS the head segmentation of arachnids Emily V. W. Settona and Prashant P. Sharmaa,1 aDepartment of Integrative Biology, University of Wisconsin–Madison, Madison, WI 53706 Edited by Nipam H. Patel, University of California, Berkeley, CA, and accepted by Editorial Board Member David Jablonski February 28, 2018 (received for review November 20, 2017) The jointed appendages of arthropods have facilitated the spec- ventral tissue. Ectopic expression of both Dmel–Sp6-9 and Dmel- tacular diversity and success of this phylum. Key to the regulation btd can induce wing-to-leg transformations, but Dmel–Sp6-9 has of appendage outgrowth is the Krüppel-like factor (KLF)/specific- a more important role in D. melanogaster leg fate specification, ity protein (Sp) family of zinc finger transcription factors. In the as Dmel–Sp6-9 can rescue double-null mutants, whereas Dmel- fruit fly, Drosophila melanogaster, the Sp6-9 homolog is activated btd cannot. In later development, both Dmel–Sp6-9 and Dmel-btd by Wnt-1/wingless (wg) and establishes ventral appendage (leg) are required for proper leg growth in larval stages (27, 28). fate. Subsequently, Sp6-9 maintains expression of the axial pat- Beyond D. melanogaster, gene expression surveys of Sp6-9 have terning gene Distal-less (Dll), which promotes limb outgrowth. In- demonstrated conservation of expression domains across insects and triguingly, in spiders, Dll has been reported to have a derived role one crustacean [all members of the same clade, Pancrustacea (29)]; as a segmentation gap gene, but the evolutionary origin and reg- taxonomically broader surveys of wg have shown broadly conserved ulation of this function are not understood because functional in- expression patterns across Arthropoda.