Acanthamoeba Keratitis: Early Diagnosis, Rational Drug Intervention and Prevention
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
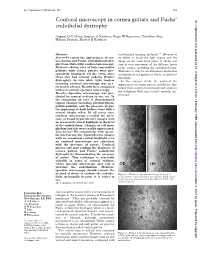
Confocal Microscopy in Cornea Guttata and Fuchs' Endothelial Dystrophy
Br J Ophthalmol 1999;83:185–189 185 Confocal microscopy in cornea guttata and Fuchs’ Br J Ophthalmol: first published as 10.1136/bjo.83.2.185 on 1 February 1999. Downloaded from endothelial dystrophy Auguste G-Y Chiou, Stephen C Kaufman, Roger W Beuerman, Toshihiko Ohta, Hisham Soliman, Herbert E Kaufman Abstract conventional imaging methods.3–13 Because of Aims—To report the appearances of cor- its ability to focus the light source and the nea guttata and Fuchs’ endothelial dystro- image on the same focal plane, it allows real phy from white light confocal microscopy. time in vivo assessment of the diVerent layers Methods—Seven eyes of four consecutive of the cornea, including the endothelial layer. patients with cornea guttata were pro- Therefore, it may be an alternative method in spectively examined. Of the seven eyes, evaluating cornea guttata or Fuchs’ endothelial three also had corneal oedema (Fuchs’ dystrophy. dystrophy). In vivo white light tandem In the current study, we analysed the scanning confocal microscopy was per- appearances of cornea guttata and Fuchs’ dys- formed in all eyes. Results were compared trophy from confocal microscopy and compare with non-contact specular microscopy. the technique with non-contact specular mi- Results—Specular microscopy was pre- croscopy. cluded by corneal oedema in one eye. In the remaining six eyes, it demonstrated typical changes including pleomorphism, polymegathism, and the presence of gut- tae appearing as dark bodies, some with a central bright reflex. In all seven eyes, confocal microscopy revealed the pres- ence of round hyporeflective images with an occasional central highlight at the level of the endothelium. -

Antiseptics and Disinfectants for the Treatment Of
Verstraelen et al. BMC Infectious Diseases 2012, 12:148 http://www.biomedcentral.com/1471-2334/12/148 RESEARCH ARTICLE Open Access Antiseptics and disinfectants for the treatment of bacterial vaginosis: A systematic review Hans Verstraelen1*, Rita Verhelst2, Kristien Roelens1 and Marleen Temmerman1,2 Abstract Background: The study objective was to assess the available data on efficacy and tolerability of antiseptics and disinfectants in treating bacterial vaginosis (BV). Methods: A systematic search was conducted by consulting PubMed (1966-2010), CINAHL (1982-2010), IPA (1970- 2010), and the Cochrane CENTRAL databases. Clinical trials were searched for by the generic names of all antiseptics and disinfectants listed in the Anatomical Therapeutic Chemical (ATC) Classification System under the code D08A. Clinical trials were considered eligible if the efficacy of antiseptics and disinfectants in the treatment of BV was assessed in comparison to placebo or standard antibiotic treatment with metronidazole or clindamycin and if diagnosis of BV relied on standard criteria such as Amsel’s and Nugent’s criteria. Results: A total of 262 articles were found, of which 15 reports on clinical trials were assessed. Of these, four randomised controlled trials (RCTs) were withheld from analysis. Reasons for exclusion were primarily the lack of standard criteria to diagnose BV or to assess cure, and control treatment not involving placebo or standard antibiotic treatment. Risk of bias for the included studies was assessed with the Cochrane Collaboration’s tool for assessing risk of bias. Three studies showed non-inferiority of chlorhexidine and polyhexamethylene biguanide compared to metronidazole or clindamycin. One RCT found that a single vaginal douche with hydrogen peroxide was slightly, though significantly less effective than a single oral dose of metronidazole. -

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

Onchocerciasis
11 ONCHOCERCIASIS ADRIAN HOPKINS AND BOAKYE A. BOATIN 11.1 INTRODUCTION the infection is actually much reduced and elimination of transmission in some areas has been achieved. Differences Onchocerciasis (or river blindness) is a parasitic disease in the vectors in different regions of Africa, and differences in cause by the filarial worm, Onchocerca volvulus. Man is the the parasite between its savannah and forest forms led to only known animal reservoir. The vector is a small black fly different presentations of the disease in different areas. of the Simulium species. The black fly breeds in well- It is probable that the disease in the Americas was brought oxygenated water and is therefore mostly associated with across from Africa by infected people during the slave trade rivers where there is fast-flowing water, broken up by catar- and found different Simulium flies, but ones still able to acts or vegetation. All populations are exposed if they live transmit the disease (3). Around 500,000 people were at risk near the breeding sites and the clinical signs of the disease in the Americas in 13 different foci, although the disease has are related to the amount of exposure and the length of time recently been eliminated from some of these foci, and there is the population is exposed. In areas of high prevalence first an ambitious target of eliminating the transmission of the signs are in the skin, with chronic itching leading to infection disease in the Americas by 2012. and chronic skin changes. Blindness begins slowly with Host factors may also play a major role in the severe skin increasingly impaired vision often leading to total loss of form of the disease called Sowda, which is found mostly in vision in young adults, in their early thirties, when they northern Sudan and in Yemen. -

Reseptregisteret 2013–2017 the Norwegian Prescription Database
LEGEMIDDELSTATISTIKK 2018:2 Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Christian Berg Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Utgitt av Folkehelseinstituttet/Published by Norwegian Institute of Public Health Område for Helsedata og digitalisering Avdeling for Legemiddelstatistikk Juni 2018 Tittel/Title: Legemiddelstatistikk 2018:2 Reseptregisteret 2013–2017 / The Norwegian Prescription Database 2013–2017 Forfattere/Authors: Christian Berg, redaktør/editor Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Acknowledgement: Julie D. W. Johansen (English text) Bestilling/Order: Rapporten kan lastes ned som pdf på Folkehelseinstituttets nettsider: www.fhi.no The report can be downloaded from www.fhi.no Grafisk design omslag: Fete Typer Ombrekking: Houston911 Kontaktinformasjon/Contact information: Folkehelseinstituttet/Norwegian Institute of Public Health Postboks 222 Skøyen N-0213 Oslo Tel: +47 21 07 70 00 ISSN: 1890-9647 ISBN: 978-82-8082-926-9 Sitering/Citation: Berg, C (red), Reseptregisteret 2013–2017 [The Norwegian Prescription Database 2013–2017] Legemiddelstatistikk 2018:2, Oslo, Norge: Folkehelseinstituttet, 2018. Tidligere utgaver / Previous editions: 2008: Reseptregisteret 2004–2007 / The Norwegian Prescription Database 2004–2007 2009: Legemiddelstatistikk 2009:2: Reseptregisteret 2004–2008 / The Norwegian Prescription Database 2004–2008 2010: Legemiddelstatistikk 2010:2: Reseptregisteret 2005–2009. Tema: Vanedannende legemidler / The Norwegian Prescription Database 2005–2009. -

Fusarium Keratitis and Corneal Collagen Cross
FUSARIUM KERATITIS AND SURGERY REFRACTIVE CORNEAL COLLAGEN CROSS-LINKING BY MINAS CORONEO, AO, BSC(MED), MBBS, MSC SYD, MD, MS, UNSW, FRACS, FRANZCO; RAJESH FOGLA, DNB, FRCS(EDIN), MMED(OPHTH); WILLIAM B. TRATTLER, MD; ASHIYANA NARIANI, MD, MPH; COMPLEX CASE MANAGEMENT COMPLEX GARGI KHARE VORA, MD; AND ALAN N. CARLSON, MD CASE PRESENTATION A 42-year-old white man is referred to the Duke University Eye Center Cornea Service for a central corneal ulcer with a hypopyon in his right eye. The patient sustained the ocular injury while mowing the lawn, with debris getting into the eye while he was wearing contact lenses. He was diagnosed with culture-positive Fusarium species by the referring ophthalmologist and was treat- ed with oral voriconazole 200 mg twice daily and frequent topical natamycin 5% and voriconazole 10 mg/mL. The patient under- went epithelium-off corneal collagen cross-linking (CXL) approxi- Figure 1. Initial evaluation of the eye with a Fusarium corneal mately 4 weeks after diagnosis of the ulcer and was treated with infiltrate and hypopyon. a loteprednol steroid taper after the procedure. His condition subsequently progressed, with increasing eye pain, a nonhealing epithelial defect, and a worsening corneal infiltrate. Upon evaluation, the patient has a large corneal infiltrate with necrotic stroma, which is approaching the limbus, and a hypopyon (Figure 1). His UCVA measures 20/70-1. B-scan ultrasound of the right eye shows no evidence of posterior segment involvement. Reculturing of the corneal infiltrate is negative for bacteria, fungus, and Acanthamoeba. Confocal microscopy reveals no evidence of hyphae or cysts. -

Immune Defense at the Ocular Surface
Eye (2003) 17, 949–956 & 2003 Nature Publishing Group All rights reserved 0950-222X/03 $25.00 www.nature.com/eye Immune defense at EK Akpek and JD Gottsch CAMBRIDGE OPHTHALMOLOGICAL SYMPOSIUM the ocular surface Abstract vertebrates. Improved visual acuity would have increased the fitness of these animals and would The ocular surface is constantly exposed to a have outweighed the disadvantage of having wide array of microorganisms. The ability of local immune cells and blood vessels at a the outer ocular system to recognize pathogens distance where a time delay in addressing a as foreign and eliminate them is critical to central corneal infection could lead to blindness. retain corneal transparency, hence The first vertebrates were jawless fish that preservation of sight. Therefore, a were believed to have evolved some 470 million combination of mechanical, anatomical, and years ago.1 These creatures had frontal eyes and immunological defense mechanisms has inhabited the shorelines of ancient oceans. With evolved to protect the outer eye. These host better vision, these creatures were likely more defense mechanisms are classified as either a active and predatory. This advantage along with native, nonspecific defense or a specifically the later development of jaws enabled bony fish acquired immunological defense requiring to flourish and establish other habitats. One previous exposure to an antigen and the such habitat was shallow waters where lunged development of specific immunity. Sight- fish made the transition to land several hundred threatening immunopathology with thousand years later.2 To become established in autologous cell damage also can take place this terrestrial environment, the new vertebrates after these reactions. -

Treatment of Interface Keratitis with Oral Corticosteroids
Treatment of interface keratitis with oral corticosteroids Scott M. MacRae, MD, Larry F. Rich, MD, Damien C. Macaluso, MD ABSTRACT Purpose: To describe the results of treating interface keratitis using a combination of intensive topical and oral corticosteroids. Setting: Casey Eye Institute, Portland, Oregon, USA. Methods: Thirteen eyes treated for grade 2 to 3 interface keratitis using an oral cortico- steroid (prednisone 60 to 80 mg) as well as an hourly topical corticosteroid were retrospectively reviewed. The best corrected visual acuity (BCVA) was used as an objective guide of whether to treat with intense topical and oral corticosteroids, flap irrigation, or both. Predisposing factors such as intraoperative epithelial defects or a history of severe allergies or atopy were also looked for. Results: All 13 eyes responded favorably to the combination of intensive topical and oral corticosteroids and had a BCVA of 20/20 after the keratitis resolved. In 6 eyes (46%), the patients had a history of severe seasonal allergies. One day postoperatively, 3 eyes (23%) had an epithelial defect and 2 eyes (15%), lint particles or debris embedded in the interface. With oral corticosteroid use, 3 patients (23%) noted mild stomach irritation and 2 (15%) noted nervousness. All 5 side effects resolved without sequelae. No patient developed a serious side effect. Conclusion: A short, intense course of an oral corticosteroid was an effective treatment in patients with grade 2 or higher interface keratitis when combined with a topical corti- costeroid administered hourly. The BCVA is a helpful objective measure of the severity of interface keratitis and can be used to guide the clinician in the therapeutic strategy. -

Frequency and Risk Factors of Symptomatic Dry Eye Disease at Tertiary Care Eye Hospital, Karachi
Biostatistics and Biometrics Open Access Journal ISSN: 2573-2633 Research Article Biostat Biometrics Open Acc J Faisal’s Issue - January 2018 Copyright © All rights are reserved by Muhammad Faisal Fahim DOI: 10.19080/BBOAJ.2018.04.555639 Frequency and Risk Factors of Symptomatic Dry Eye Disease at Tertiary Care Eye Hospital, Karachi Shaheerah Gul1, Adil Salim Jafri1, Muhammad Faisal Fahim2* 1Department of Ophthalmology, Al-Ibrahim Eye Hospital, Pakistan 2Department of Research & Development, Al-Ibrahim Eye Hospital, Pakistan Submission: November 27, 2017; Published: January 19, 2018 *Corresponding author: Muhammad Faisal Fahim, M.Sc (Statistics), Statistician, Research & Development Department, Al-Ibrahim Eye Hospital, Isra postgraduate Institute of Ophthalmology, Karachi, Pakistan, Tel: ; Email: Abstract Objective: To determine frequency and risk factors of symptomatic dry eye disease at tertiary care eye hospital, Karachi. Material & Methods: This was a descriptive cross sectional study carried out at Al-Ibrahim Eye Hospital, Isra postgraduate Institute of Oph- thalmology, Karachi from March to October 2016. Non-Probability purposive sampling technique was used for data collection. Inclusion criteria give consent. Symptoms of dry eye were assessed using Tear breakup test (TBUT) test. SPSS version 20.0 was used to analyze data. were patients aged ≥ 21 years and on the basis of dry eye symptoms. Exclusion criteria were other systemic eye disease and those who did not Results: A total of 100 patients 65 female and 35 male were diagnosed with dry eye syndrome. The age group of 21-30 years having the high- est frequency of 34 patients, whereas after the 50 years of age the frequency of patients decreases to 21. -

Reactive Uveitis, Retinal Vasculitis and Scleritis As Ocular End-Stage of Acanthamoeba Keratitis: a Histological Study
Uveitis and vasculitis of Acanthamoeba keratitis ·Brief Report· Reactive uveitis, retinal vasculitis and scleritis as ocular end-stage of Acanthamoeba keratitis: a histological study Lei Shi1,2, Tobias Hager1, Fabian Norbert Fries1, Loay Daas1, Leonard Holbach3, Carmen Hofmann- Rummelt3, Elena Zemova1, Berthold Seitz1, Nóra Szentmáry1,4 1Department of Ophthalmology, Saarland University Medical wearers. Its annual incidence was 17.53 to 21.14 per one Center, Homburg/Saar 66424, Germany million contact lens wearers in the UK[1]. In Germany, with 2Department of Ophthalmology, The First Affiliated Hospital about 80 million inhabitants, about 150 new cases have been of USTC, Division of Life Sciences and Medicine, University reported in a 10-year-period[2]. Studies showed that 68%-92.3% of Science and Technology of China, Hefei 230001, Anhui of AK patients are contact lens wearers[1,3-4]. Expression of Province, P.R. China mannosilated glycoproteins on corneal epithelial cell surface is 3Department of Ophthalmology, Friedrich-Alexander upregulated in contact lens wearers[3]. This plays an important University Erlangen-Nürnberg, Erlangen 91052, Germany role in AK pathogenesis. The Acanthamoeba trophozoite binds 4Department of Ophthalmology, Semmelweis University, to these proteins though its mannose-binding site in order to Budapest 1093, Hungary release the so-called mannose-induced protease 133 (MIP-133) Correspondence to: Lei Shi. Department of Ophthalmology, and Acanthamoeba plasminogen activator (aPA). MIP-133 and Saarland University Medical Center, Homburg/Saar, Kirrberger aPA give rise to lysis of epithelial, stromal cells and stromal Str. 100. 66424, Germany. [email protected] matrix, leading to corneal erosions and ulceration[4]. Presence Received: 2019-05-01 Accepted: 2019-08-21 of bacteria or fungi also supports Acanthamoeba growth, often resulting in co-infection[5]. -

Acanthamoeba, Fungal, and Bacterial Keratitis: a Comparison of Risk Factors and Clinical Features
Acanthamoeba, Fungal, and Bacterial Keratitis: A Comparison of Risk Factors and Clinical Features JEENA MASCARENHAS, PRAJNA LALITHA, N. VENKATESH PRAJNA, MUTHIAH SRINIVASAN, MANORANJAN DAS, SEAN S. D’SILVA, CATHERINE E. OLDENBURG, DURGA S. BORKAR, ELIZABETH J. ESTERBERG, THOMAS M. LIETMAN, AND JEREMY D. KEENAN PURPOSE: To determine risk factors and clinical signs acanthamoeba keratitis research has been conducted in that may differentiate between bacterial, fungal, and industrialized countries, acanthamoeba keratitis also acanthamoeba keratitis among patients presenting with occurs in developing countries, often in non–contact presumed infectious keratitis. lens–wearing individuals.6,7 DESIGN: Hospital-based cross-sectional study. Acanthamoeba keratitis is frequently misdiagnosed as METHODS: We examined the medical records of 115 herpetic or fungal keratitis, and is subsequently treated incor- patients with laboratory-proven bacterial keratitis, 115 rectly, which can lead to poor outcomes.8 Case series of acan- patients with laboratory-proven fungal keratitis, and thamoeba keratitis have identified several important clinical 115 patients with laboratory-proven acanthamoeba kera- signs, such as pseudodendrites, perineural infiltrates, and ring titis seen at Aravind Eye Hospital, Madurai, India, from infiltrates.9,10 However, we are unaware of any studies that 2006-2011. Risk factors and clinical features of the 3 have compared the clinical findings of acanthamoeba organisms were compared using multinomial logistic keratitis with those of bacterial and fungal keratitis. regression. Clinical signs can be especially useful for differentiating the RESULTS: Of 95 patients with bacterial keratitis, 103 cause of infectious keratitis when microbiological testing is patients with fungal keratitis, and 93 patients with acan- not available—which is frequently the case in developing thamoeba keratitis who had medical records available for countries. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01