Developing Novel Therapeutic Agents for Acanthamoeba Infection and Investigating the Process of Encystment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
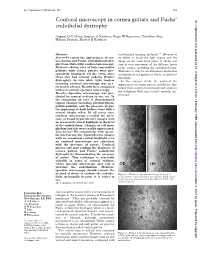
Confocal Microscopy in Cornea Guttata and Fuchs' Endothelial Dystrophy
Br J Ophthalmol 1999;83:185–189 185 Confocal microscopy in cornea guttata and Fuchs’ Br J Ophthalmol: first published as 10.1136/bjo.83.2.185 on 1 February 1999. Downloaded from endothelial dystrophy Auguste G-Y Chiou, Stephen C Kaufman, Roger W Beuerman, Toshihiko Ohta, Hisham Soliman, Herbert E Kaufman Abstract conventional imaging methods.3–13 Because of Aims—To report the appearances of cor- its ability to focus the light source and the nea guttata and Fuchs’ endothelial dystro- image on the same focal plane, it allows real phy from white light confocal microscopy. time in vivo assessment of the diVerent layers Methods—Seven eyes of four consecutive of the cornea, including the endothelial layer. patients with cornea guttata were pro- Therefore, it may be an alternative method in spectively examined. Of the seven eyes, evaluating cornea guttata or Fuchs’ endothelial three also had corneal oedema (Fuchs’ dystrophy. dystrophy). In vivo white light tandem In the current study, we analysed the scanning confocal microscopy was per- appearances of cornea guttata and Fuchs’ dys- formed in all eyes. Results were compared trophy from confocal microscopy and compare with non-contact specular microscopy. the technique with non-contact specular mi- Results—Specular microscopy was pre- croscopy. cluded by corneal oedema in one eye. In the remaining six eyes, it demonstrated typical changes including pleomorphism, polymegathism, and the presence of gut- tae appearing as dark bodies, some with a central bright reflex. In all seven eyes, confocal microscopy revealed the pres- ence of round hyporeflective images with an occasional central highlight at the level of the endothelium. -

(12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao Et Al
USOO9498481 B2 (12) United States Patent (10) Patent No.: US 9,498,481 B2 Rao et al. (45) Date of Patent: *Nov. 22, 2016 (54) CYCLOPROPYL MODULATORS OF P2Y12 WO WO95/26325 10, 1995 RECEPTOR WO WO99/O5142 2, 1999 WO WOOO/34283 6, 2000 WO WO O1/92262 12/2001 (71) Applicant: Apharaceuticals. Inc., La WO WO O1/922.63 12/2001 olla, CA (US) WO WO 2011/O17108 2, 2011 (72) Inventors: Tadimeti Rao, San Diego, CA (US); Chengzhi Zhang, San Diego, CA (US) OTHER PUBLICATIONS Drugs of the Future 32(10), 845-853 (2007).* (73) Assignee: Auspex Pharmaceuticals, Inc., LaJolla, Tantry et al. in Expert Opin. Invest. Drugs (2007) 16(2):225-229.* CA (US) Wallentin et al. in the New England Journal of Medicine, 361 (11), 1045-1057 (2009).* (*) Notice: Subject to any disclaimer, the term of this Husted et al. in The European Heart Journal 27, 1038-1047 (2006).* patent is extended or adjusted under 35 Auspex in www.businesswire.com/news/home/20081023005201/ U.S.C. 154(b) by Od en/Auspex-Pharmaceuticals-Announces-Positive-Results-Clinical M YW- (b) by ayS. Study (published: Oct. 23, 2008).* This patent is Subject to a terminal dis- Concert In www.concertpharma. com/news/ claimer ConcertPresentsPreclinicalResultsNAMS.htm (published: Sep. 25. 2008).* Concert2 in Expert Rev. Anti Infect. Ther. 6(6), 782 (2008).* (21) Appl. No.: 14/977,056 Springthorpe et al. in Bioorganic & Medicinal Chemistry Letters 17. 6013-6018 (2007).* (22) Filed: Dec. 21, 2015 Leis et al. in Current Organic Chemistry 2, 131-144 (1998).* Angiolillo et al., Pharmacology of emerging novel platelet inhibi (65) Prior Publication Data tors, American Heart Journal, 2008, 156(2) Supp. -

Entamoeba Histolytica
Journal of Clinical Microbiology and Biochemical Technology Piotr Nowak1*, Katarzyna Mastalska1 Review Article and Jakub Loster2 1Laboratory of Parasitology, Department of Microbiology, University Hospital in Krakow, 19 Entamoeba Histolytica - Pathogenic Kopernika Street, 31-501 Krakow, Poland 2Department of Infectious Diseases, University Protozoan of the Large Intestine in Hospital in Krakow, 5 Sniadeckich Street, 31-531 Krakow, Poland Humans Dates: Received: 01 December, 2015; Accepted: 29 December, 2015; Published: 30 December, 2015 *Corresponding author: Piotr Nowak, Laboratory of Abstract Parasitology, Department of Microbiology, University Entamoeba histolytica is a cosmopolitan, parasitic protozoan of human large intestine, which is Hospital in Krakow, 19 Kopernika Street, 31- 501 a causative agent of amoebiasis. Amoebiasis manifests with persistent diarrhea containing mucus Krakow, Poland, Tel: +4812/4247587; Fax: +4812/ or blood, accompanied by abdominal pain, flatulence, nausea and fever. In some cases amoebas 4247581; E-mail: may travel through the bloodstream from the intestine to the liver or to other organs, causing multiple www.peertechz.com abscesses. Amoebiasis is a dangerous, parasitic disease and after malaria the second cause of deaths related to parasitic infections worldwide. The highest rate of infections is observed among people living Keywords: Entamoeba histolytica; Entamoeba in or traveling through the tropics. Laboratory diagnosis of amoebiasis is quite difficult, comprising dispar; Entamoeba moshkovskii; Entamoeba of microscopy and methods of molecular biology. Pathogenic species Entamoeba histolytica has to histolytica sensu lato; Entamoeba histolytica sensu be differentiated from other nonpathogenic amoebas of the intestine, so called commensals, that stricto; commensals of the large intestine; amoebiasis very often live in the human large intestine and remain harmless. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

A Revised Classification of Naked Lobose Amoebae (Amoebozoa
Protist, Vol. 162, 545–570, October 2011 http://www.elsevier.de/protis Published online date 28 July 2011 PROTIST NEWS A Revised Classification of Naked Lobose Amoebae (Amoebozoa: Lobosa) Introduction together constitute the amoebozoan subphy- lum Lobosa, which never have cilia or flagella, Molecular evidence and an associated reevaluation whereas Variosea (as here revised) together with of morphology have recently considerably revised Mycetozoa and Archamoebea are now grouped our views on relationships among the higher-level as the subphylum Conosa, whose constituent groups of amoebae. First of all, establishing the lineages either have cilia or flagella or have lost phylum Amoebozoa grouped all lobose amoe- them secondarily (Cavalier-Smith 1998, 2009). boid protists, whether naked or testate, aerobic Figure 1 is a schematic tree showing amoebozoan or anaerobic, with the Mycetozoa and Archamoe- relationships deduced from both morphology and bea (Cavalier-Smith 1998), and separated them DNA sequences. from both the heterolobosean amoebae (Page and The first attempt to construct a congruent molec- Blanton 1985), now belonging in the phylum Per- ular and morphological system of Amoebozoa by colozoa - Cavalier-Smith and Nikolaev (2008), and Cavalier-Smith et al. (2004) was limited by the the filose amoebae that belong in other phyla lack of molecular data for many amoeboid taxa, (notably Cercozoa: Bass et al. 2009a; Howe et al. which were therefore classified solely on morpho- 2011). logical evidence. Smirnov et al. (2005) suggested The phylum Amoebozoa consists of naked and another system for naked lobose amoebae only; testate lobose amoebae (e.g. Amoeba, Vannella, this left taxa with no molecular data incertae sedis, Hartmannella, Acanthamoeba, Arcella, Difflugia), which limited its utility. -

Autonomic Nervous System Final.Pdf
University of Hawai‘i Hilo Pre- Nursing Program NURS 203 – General AUTONOMIC NERVOUS SYSTEM Pharmacology Danita Narciso Pharm D 1 LEARNING OBJECTIVES Understand the basic function of the autonomic nervous system (ANS) Know the neurotransmitters and receptors of each branch of the autonomic nervous system Understand if a tissue or organ is being activated by a certain branch of the ANS what the resulting action would be Understand how these two systems work in concert for daily living and situation of fight or flight 2 AUTONOMIC NERVOUS SYSTEM – WHERE IT FITS IN Nervous System Central Peripheral Somatic Autonomic (Skeletal Muscle) Parasympathetic Sympathetic Brain (cholinergic) ACh (adrenergic) NE Spinal Cord Nicotinic Alpha Beta α1, α2 β1, β2 Muscarinic 3 AUTONOMIC NERVOUS SYSTEM Fight or Flight Adrenergic Rest & Digest Norepinephrine Cholinergic Acetylcholine 4 AUTONOMIC NERVOUS SYSTEM – WHERE IT FITS IN Nervous System Central Peripheral Somatic Autonomic (Skeletal Muscle) Parasympathetic Sympathetic Brain (cholinergic) ACh (adrenergic) NE Spinal Cord Nicotinic Alpha Beta α1, α2 β1, β2 Muscarinic 5 AUTONOMIC NERVOUS SYSTEM (ANS) Parasympathetic NS Nicotinic Muscarinic Peripheral Sympathetic NS Alpha Autonomic Beta Nerves Parasympathetic Sympathetic Carrying ACh (cholinergic) ACh (adrenergic) NE Cholinergic Carrying NE Nicotinic Beta Adrenergic Alpha α1, α2 β1, β2 Muscarinic 6 AUTONOMIC NERVOUS SYSTEM (ANS) 7 AUTONOMIC NERVOUS SYSTEM (ANS) Ganglion: Group of nerve cell bodies. Connects pre and post ganglionic nerves. 8 AUTONOMIC NERVOUS SYSTEM (ANS) 9 AUTONOMIC NERVOUS SYSTEM (ANS) 10 AUTONOMIC NERVOUS SYSTEM (ANS) Preganglionic PNS ACh Preganglionic SNS ACh Post ganglionic Post ganglionic PNS SNS ACh NE 11 ACTIONS OF THE ANS - SNS Think fight or flight Dilate pupils Let in more light to see the bear Inhibit salivation This is no time to be hungry Relax airways Increase O2 intake Increase heart rate …. -

Multiple Roots of Fruiting Body Formation in Amoebozoa
GBE Multiple Roots of Fruiting Body Formation in Amoebozoa Falk Hillmann1,*, Gillian Forbes2, Silvia Novohradska1, Iuliia Ferling1,KonstantinRiege3,MarcoGroth4, Martin Westermann5,ManjaMarz3, Thomas Spaller6, Thomas Winckler6, Pauline Schaap2,and Gernot Glo¨ ckner7,* 1Junior Research Group Evolution of Microbial Interaction, Leibniz Institute for Natural Product Research and Infection Biology – Hans Kno¨ ll Institute (HKI), Jena, Germany 2Division of Cell and Developmental Biology, School of Life Sciences, University of Dundee, United Kingdom 3Bioinformatics/High Throughput Analysis, Friedrich Schiller University Jena, Germany 4CF DNA-Sequencing, Leibniz Institute on Aging Research, Jena, Germany 5Electron Microscopy Center, Jena University Hospital, Germany 6Pharmaceutical Biology, Institute of Pharmacy, Friedrich Schiller University Jena, Germany 7Institute of Biochemistry I, Medical Faculty, University of Cologne, Germany *Corresponding authors: E-mails: [email protected]; [email protected]. Accepted: January 11, 2018 Data deposition: The genome sequence and gene predictions of Protostelium aurantium and Protostelium mycophagum were deposited in GenBank under the Accession Numbers MDYQ00000000 and MZNV00000000, respectively. The mitochondrial genome of P. mycophagum was deposited under the Accession number KY75056 and that of P. aurantium under the Accession number KY75057. The RNAseq reads can be found in Bioproject Accession PRJNA338377. All sequence and annotation data are also available directly from the authors. The P. aurantium strain is deposited in the Jena Microbial Resource Collection (JMRC) under accession number SF0012540. Abstract Establishment of multicellularity represents a major transition in eukaryote evolution. A subgroup of Amoebozoa, the dictyos- teliids, has evolved a relatively simple aggregative multicellular stage resulting in a fruiting body supported by a stalk. Protosteloid amoeba, which are scattered throughout the amoebozoan tree, differ by producing only one or few single stalked spores. -

Fusarium Keratitis and Corneal Collagen Cross
FUSARIUM KERATITIS AND SURGERY REFRACTIVE CORNEAL COLLAGEN CROSS-LINKING BY MINAS CORONEO, AO, BSC(MED), MBBS, MSC SYD, MD, MS, UNSW, FRACS, FRANZCO; RAJESH FOGLA, DNB, FRCS(EDIN), MMED(OPHTH); WILLIAM B. TRATTLER, MD; ASHIYANA NARIANI, MD, MPH; COMPLEX CASE MANAGEMENT COMPLEX GARGI KHARE VORA, MD; AND ALAN N. CARLSON, MD CASE PRESENTATION A 42-year-old white man is referred to the Duke University Eye Center Cornea Service for a central corneal ulcer with a hypopyon in his right eye. The patient sustained the ocular injury while mowing the lawn, with debris getting into the eye while he was wearing contact lenses. He was diagnosed with culture-positive Fusarium species by the referring ophthalmologist and was treat- ed with oral voriconazole 200 mg twice daily and frequent topical natamycin 5% and voriconazole 10 mg/mL. The patient under- went epithelium-off corneal collagen cross-linking (CXL) approxi- Figure 1. Initial evaluation of the eye with a Fusarium corneal mately 4 weeks after diagnosis of the ulcer and was treated with infiltrate and hypopyon. a loteprednol steroid taper after the procedure. His condition subsequently progressed, with increasing eye pain, a nonhealing epithelial defect, and a worsening corneal infiltrate. Upon evaluation, the patient has a large corneal infiltrate with necrotic stroma, which is approaching the limbus, and a hypopyon (Figure 1). His UCVA measures 20/70-1. B-scan ultrasound of the right eye shows no evidence of posterior segment involvement. Reculturing of the corneal infiltrate is negative for bacteria, fungus, and Acanthamoeba. Confocal microscopy reveals no evidence of hyphae or cysts. -

Reactive Uveitis, Retinal Vasculitis and Scleritis As Ocular End-Stage of Acanthamoeba Keratitis: a Histological Study
Uveitis and vasculitis of Acanthamoeba keratitis ·Brief Report· Reactive uveitis, retinal vasculitis and scleritis as ocular end-stage of Acanthamoeba keratitis: a histological study Lei Shi1,2, Tobias Hager1, Fabian Norbert Fries1, Loay Daas1, Leonard Holbach3, Carmen Hofmann- Rummelt3, Elena Zemova1, Berthold Seitz1, Nóra Szentmáry1,4 1Department of Ophthalmology, Saarland University Medical wearers. Its annual incidence was 17.53 to 21.14 per one Center, Homburg/Saar 66424, Germany million contact lens wearers in the UK[1]. In Germany, with 2Department of Ophthalmology, The First Affiliated Hospital about 80 million inhabitants, about 150 new cases have been of USTC, Division of Life Sciences and Medicine, University reported in a 10-year-period[2]. Studies showed that 68%-92.3% of Science and Technology of China, Hefei 230001, Anhui of AK patients are contact lens wearers[1,3-4]. Expression of Province, P.R. China mannosilated glycoproteins on corneal epithelial cell surface is 3Department of Ophthalmology, Friedrich-Alexander upregulated in contact lens wearers[3]. This plays an important University Erlangen-Nürnberg, Erlangen 91052, Germany role in AK pathogenesis. The Acanthamoeba trophozoite binds 4Department of Ophthalmology, Semmelweis University, to these proteins though its mannose-binding site in order to Budapest 1093, Hungary release the so-called mannose-induced protease 133 (MIP-133) Correspondence to: Lei Shi. Department of Ophthalmology, and Acanthamoeba plasminogen activator (aPA). MIP-133 and Saarland University Medical Center, Homburg/Saar, Kirrberger aPA give rise to lysis of epithelial, stromal cells and stromal Str. 100. 66424, Germany. [email protected] matrix, leading to corneal erosions and ulceration[4]. Presence Received: 2019-05-01 Accepted: 2019-08-21 of bacteria or fungi also supports Acanthamoeba growth, often resulting in co-infection[5]. -

General Prescription
GENERAL PRESCRIPTION LESSON 1. INTRODUCTION. PRESCRIPTION. SOLID MEDICINAL FORMS Objective: To study the structure of the prescription, learn the rules and get practical skills in writing out solid medicinal forms in prescription. To carry out practical tasks on prescriptions it is recommended to use Appendix 1. Key questions: 1. Pharmacology as a science and the basis of therapy. Main development milestones of modern pharmacology. Sections of Pharmacology. 2. The concept of medicinal substance, medicinal agent (medicinal drug, drug), medicinal form. 3. The concept of the pharmacological action and types of the action of drugs. 4. The sources of obtaining drugs. 5. International and national pharmacopeia, its content and purpose. 6. Pharmacy. Rules of drug storage and dispensing. 7. Prescription and its structure. Prescription forms. General rules for writing out a prescription. State regulation of writing out and dispensing drugs. 8. Name of medicinal products (international non-proprietary name - INN, trade name). 9. Peculiarities of writing out narcotic, poisonous and potent substances in prescription. 10. Drugs under control. Drugs prohibited for prescribing. 11. Solid medicinal forms: tablets, dragee (pills), powders, capsules. Their characteristics, advantages and disadvantages. Rules of prescribing. Write out prescriptions for: 1. 5 powders of Codeine 0.015 g. 1 powder orally twice a day. 2. 10 powders of Didanosine 0.25 g in sachets to prepare solution for internal use. Accept inside twice a day one sachet powder after dissolution in a glass of boiled water. 3. 50 mg of Alteplase powder in the bottle. Dissolve the content of the bottle in 50 ml of saline. First 15 ml introduce intravenously streamly, then intravenous drip. -

Drug-Facilitated Sexual Assault Panel, Blood
DRUG-FACILITATED SEXUAL ASSAULT PANEL, BLOOD Blood Specimens (Order Code 70500) Alcohols Analgesics, cont. Anticonvulsants, cont. Antihistamines, cont. Ethanol Phenylbutazone Phenytoin Cyclizine Amphetamines Piroxicam Pregabalin Diphenhydramine Amphetamine Salicylic Acid* Primidone Doxylamine BDB Sulindac* Topiramate Fexofenadine Benzphetamine Tapentadol Zonisamide Guaifenesin Ephedrine Tizanidine Antidepressants Hydroxyzine MDA Tolmetin Amitriptyline Loratadine MDMA Tramadol Amoxapine Oxymetazoline* Mescaline* Anesthetics Bupropion Pyrilamine Methcathinone Benzocaine Citalopram Tetrahydrozoline Methamphetamine Bupivacaine Clomipramine Triprolidine Phentermine Etomidate Desipramine Antipsychotics PMA Ketamine Desmethylclomipramine 9-hydroxyrisperidone Phenylpropanolamine Lidocaine Dosulepin Aripiprazole Pseudoephedrine Mepivacaine Doxepin Buspirone Analgesics Methoxetamine Duloxetine Chlorpromazine Acetaminophen Midazolam Fluoxetine Clozapine Baclofen Norketamine Fluvoxamine Fluphenazine Buprenorphine Pramoxine* Imipramine Haloperidol Carisoprodol Procaine 1,3-chlorophenylpiperazine (mCPP) Mesoridazine Cyclobenzaprine Rocuronium Mianserin* Norclozapine Diclofenac Ropivacaine Mirtazapine Olanzapine Etodolac Antibiotics Nefazodone Perphenazine Fenoprofen Azithromycin* Nordoxepin Pimozide Hydroxychloroquine Chloramphenicol* Norfluoxetine Prochlorperazine Ibuprofen Ciprofloxacin* Norsertraline Quetiapine Ketoprofen Clindamycin* Nortriptyline Risperidone Ketorolac Erythromycin* Norvenlafaxine Thioridazine Meclofenamic Acid* Levofloxacin* Paroxetine -

Acanthamoeba, Fungal, and Bacterial Keratitis: a Comparison of Risk Factors and Clinical Features
Acanthamoeba, Fungal, and Bacterial Keratitis: A Comparison of Risk Factors and Clinical Features JEENA MASCARENHAS, PRAJNA LALITHA, N. VENKATESH PRAJNA, MUTHIAH SRINIVASAN, MANORANJAN DAS, SEAN S. D’SILVA, CATHERINE E. OLDENBURG, DURGA S. BORKAR, ELIZABETH J. ESTERBERG, THOMAS M. LIETMAN, AND JEREMY D. KEENAN PURPOSE: To determine risk factors and clinical signs acanthamoeba keratitis research has been conducted in that may differentiate between bacterial, fungal, and industrialized countries, acanthamoeba keratitis also acanthamoeba keratitis among patients presenting with occurs in developing countries, often in non–contact presumed infectious keratitis. lens–wearing individuals.6,7 DESIGN: Hospital-based cross-sectional study. Acanthamoeba keratitis is frequently misdiagnosed as METHODS: We examined the medical records of 115 herpetic or fungal keratitis, and is subsequently treated incor- patients with laboratory-proven bacterial keratitis, 115 rectly, which can lead to poor outcomes.8 Case series of acan- patients with laboratory-proven fungal keratitis, and thamoeba keratitis have identified several important clinical 115 patients with laboratory-proven acanthamoeba kera- signs, such as pseudodendrites, perineural infiltrates, and ring titis seen at Aravind Eye Hospital, Madurai, India, from infiltrates.9,10 However, we are unaware of any studies that 2006-2011. Risk factors and clinical features of the 3 have compared the clinical findings of acanthamoeba organisms were compared using multinomial logistic keratitis with those of bacterial and fungal keratitis. regression. Clinical signs can be especially useful for differentiating the RESULTS: Of 95 patients with bacterial keratitis, 103 cause of infectious keratitis when microbiological testing is patients with fungal keratitis, and 93 patients with acan- not available—which is frequently the case in developing thamoeba keratitis who had medical records available for countries.