Toxic Effects of Selected Proprietary Dry Eye Drops on Acanthamoeba
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
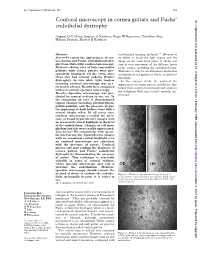
Confocal Microscopy in Cornea Guttata and Fuchs' Endothelial Dystrophy
Br J Ophthalmol 1999;83:185–189 185 Confocal microscopy in cornea guttata and Fuchs’ Br J Ophthalmol: first published as 10.1136/bjo.83.2.185 on 1 February 1999. Downloaded from endothelial dystrophy Auguste G-Y Chiou, Stephen C Kaufman, Roger W Beuerman, Toshihiko Ohta, Hisham Soliman, Herbert E Kaufman Abstract conventional imaging methods.3–13 Because of Aims—To report the appearances of cor- its ability to focus the light source and the nea guttata and Fuchs’ endothelial dystro- image on the same focal plane, it allows real phy from white light confocal microscopy. time in vivo assessment of the diVerent layers Methods—Seven eyes of four consecutive of the cornea, including the endothelial layer. patients with cornea guttata were pro- Therefore, it may be an alternative method in spectively examined. Of the seven eyes, evaluating cornea guttata or Fuchs’ endothelial three also had corneal oedema (Fuchs’ dystrophy. dystrophy). In vivo white light tandem In the current study, we analysed the scanning confocal microscopy was per- appearances of cornea guttata and Fuchs’ dys- formed in all eyes. Results were compared trophy from confocal microscopy and compare with non-contact specular microscopy. the technique with non-contact specular mi- Results—Specular microscopy was pre- croscopy. cluded by corneal oedema in one eye. In the remaining six eyes, it demonstrated typical changes including pleomorphism, polymegathism, and the presence of gut- tae appearing as dark bodies, some with a central bright reflex. In all seven eyes, confocal microscopy revealed the pres- ence of round hyporeflective images with an occasional central highlight at the level of the endothelium. -

Fusarium Keratitis and Corneal Collagen Cross
FUSARIUM KERATITIS AND SURGERY REFRACTIVE CORNEAL COLLAGEN CROSS-LINKING BY MINAS CORONEO, AO, BSC(MED), MBBS, MSC SYD, MD, MS, UNSW, FRACS, FRANZCO; RAJESH FOGLA, DNB, FRCS(EDIN), MMED(OPHTH); WILLIAM B. TRATTLER, MD; ASHIYANA NARIANI, MD, MPH; COMPLEX CASE MANAGEMENT COMPLEX GARGI KHARE VORA, MD; AND ALAN N. CARLSON, MD CASE PRESENTATION A 42-year-old white man is referred to the Duke University Eye Center Cornea Service for a central corneal ulcer with a hypopyon in his right eye. The patient sustained the ocular injury while mowing the lawn, with debris getting into the eye while he was wearing contact lenses. He was diagnosed with culture-positive Fusarium species by the referring ophthalmologist and was treat- ed with oral voriconazole 200 mg twice daily and frequent topical natamycin 5% and voriconazole 10 mg/mL. The patient under- went epithelium-off corneal collagen cross-linking (CXL) approxi- Figure 1. Initial evaluation of the eye with a Fusarium corneal mately 4 weeks after diagnosis of the ulcer and was treated with infiltrate and hypopyon. a loteprednol steroid taper after the procedure. His condition subsequently progressed, with increasing eye pain, a nonhealing epithelial defect, and a worsening corneal infiltrate. Upon evaluation, the patient has a large corneal infiltrate with necrotic stroma, which is approaching the limbus, and a hypopyon (Figure 1). His UCVA measures 20/70-1. B-scan ultrasound of the right eye shows no evidence of posterior segment involvement. Reculturing of the corneal infiltrate is negative for bacteria, fungus, and Acanthamoeba. Confocal microscopy reveals no evidence of hyphae or cysts. -

Reactive Uveitis, Retinal Vasculitis and Scleritis As Ocular End-Stage of Acanthamoeba Keratitis: a Histological Study
Uveitis and vasculitis of Acanthamoeba keratitis ·Brief Report· Reactive uveitis, retinal vasculitis and scleritis as ocular end-stage of Acanthamoeba keratitis: a histological study Lei Shi1,2, Tobias Hager1, Fabian Norbert Fries1, Loay Daas1, Leonard Holbach3, Carmen Hofmann- Rummelt3, Elena Zemova1, Berthold Seitz1, Nóra Szentmáry1,4 1Department of Ophthalmology, Saarland University Medical wearers. Its annual incidence was 17.53 to 21.14 per one Center, Homburg/Saar 66424, Germany million contact lens wearers in the UK[1]. In Germany, with 2Department of Ophthalmology, The First Affiliated Hospital about 80 million inhabitants, about 150 new cases have been of USTC, Division of Life Sciences and Medicine, University reported in a 10-year-period[2]. Studies showed that 68%-92.3% of Science and Technology of China, Hefei 230001, Anhui of AK patients are contact lens wearers[1,3-4]. Expression of Province, P.R. China mannosilated glycoproteins on corneal epithelial cell surface is 3Department of Ophthalmology, Friedrich-Alexander upregulated in contact lens wearers[3]. This plays an important University Erlangen-Nürnberg, Erlangen 91052, Germany role in AK pathogenesis. The Acanthamoeba trophozoite binds 4Department of Ophthalmology, Semmelweis University, to these proteins though its mannose-binding site in order to Budapest 1093, Hungary release the so-called mannose-induced protease 133 (MIP-133) Correspondence to: Lei Shi. Department of Ophthalmology, and Acanthamoeba plasminogen activator (aPA). MIP-133 and Saarland University Medical Center, Homburg/Saar, Kirrberger aPA give rise to lysis of epithelial, stromal cells and stromal Str. 100. 66424, Germany. [email protected] matrix, leading to corneal erosions and ulceration[4]. Presence Received: 2019-05-01 Accepted: 2019-08-21 of bacteria or fungi also supports Acanthamoeba growth, often resulting in co-infection[5]. -

Acanthamoeba, Fungal, and Bacterial Keratitis: a Comparison of Risk Factors and Clinical Features
Acanthamoeba, Fungal, and Bacterial Keratitis: A Comparison of Risk Factors and Clinical Features JEENA MASCARENHAS, PRAJNA LALITHA, N. VENKATESH PRAJNA, MUTHIAH SRINIVASAN, MANORANJAN DAS, SEAN S. D’SILVA, CATHERINE E. OLDENBURG, DURGA S. BORKAR, ELIZABETH J. ESTERBERG, THOMAS M. LIETMAN, AND JEREMY D. KEENAN PURPOSE: To determine risk factors and clinical signs acanthamoeba keratitis research has been conducted in that may differentiate between bacterial, fungal, and industrialized countries, acanthamoeba keratitis also acanthamoeba keratitis among patients presenting with occurs in developing countries, often in non–contact presumed infectious keratitis. lens–wearing individuals.6,7 DESIGN: Hospital-based cross-sectional study. Acanthamoeba keratitis is frequently misdiagnosed as METHODS: We examined the medical records of 115 herpetic or fungal keratitis, and is subsequently treated incor- patients with laboratory-proven bacterial keratitis, 115 rectly, which can lead to poor outcomes.8 Case series of acan- patients with laboratory-proven fungal keratitis, and thamoeba keratitis have identified several important clinical 115 patients with laboratory-proven acanthamoeba kera- signs, such as pseudodendrites, perineural infiltrates, and ring titis seen at Aravind Eye Hospital, Madurai, India, from infiltrates.9,10 However, we are unaware of any studies that 2006-2011. Risk factors and clinical features of the 3 have compared the clinical findings of acanthamoeba organisms were compared using multinomial logistic keratitis with those of bacterial and fungal keratitis. regression. Clinical signs can be especially useful for differentiating the RESULTS: Of 95 patients with bacterial keratitis, 103 cause of infectious keratitis when microbiological testing is patients with fungal keratitis, and 93 patients with acan- not available—which is frequently the case in developing thamoeba keratitis who had medical records available for countries. -

(RGP) Contact Lens Induced Microbial Keratitis in a Keratoconus Patient: a Case Report
IOSR Journal of Dental and Medical Sciences (JDMS) ISSN: 2279-0853, ISBN: 2279-0861. Volume 1, Issue 5 (Sep-Oct. 2012), PP 12-16 www.iosrjournals.org Rigid Gas permeable (RGP) contact lens induced microbial keratitis in a keratoconus patient: A case report. Krishnendu Mandal. M.OPT1, Bhupesh Bagga. FRCS2, 3 Sobia Taureen. B. OPT 1,3 Optometry, L.V.Prasad Eye Institute, india 2Ophthalmology, L.V.Prasad Eye Institute, india ABSTRACT : Introduction: To report a case of microbial keratitis in an individual with keratoconus using rigid gas permeable contact lenses. Method: - A 23- year-old male presented with a history of pain, redness, photophobia, watering in right eye and his vision was reduced upon awakening. He was a known case of bilateral keratoconus who used rigid gas permeable contact lenses in both eyes on daily wear basis. Slitlamp examination revealed a paracentral stromal infiltrate in right eye. Corneal scrapings were collected for culture. Both contact lenses and lenses cleaning solution were collected for microbiological investigations. Result: - Corneal scraping revealed plenty gram-negative bacteria (Pseudomonas aeruginosa) and contact lenses cleaning solution revealed klebsiella. Conclusion:- Although microbial keratitis is an uncommon complication with rigid gas permeable contact lenses in keratoconus patients but it can be managed by proper microbiological work up and intensive medical care. Key words:KC-Keratoconus, RGP- rigid gas permeable contact lens, MK-Microbial keratitis, Pseudomonas aeruginosa, Klebsiella. I. Introduction -

Acanthamoeba Keratitis:10.5005/Jp-Journals-10025-1125 Different Surgical Approaches Case Series
IJKECD Acanthamoeba Keratitis:10.5005/jp-journals-10025-1125 Different Surgical Approaches CASE SERIES Acanthamoeba Keratitis: Different Surgical Approaches 1Mukharram Bikbov, 2Valentina Surkova, 3Emin Usubov ABSTRACT sensitive layer of the cornea. The diagnosis deemed to be The features of acanthamoeba keratitis (AK) progression, complete only when cysts are detected in the material clinical cases, and results of early and delayed penetrating taken from the cornea and its agar inoculation, smears keratoplasty as the main method of severe AK treatment are of CLs, and their cases. presented. It is described as two clinical cases with different The AK conservative treatment is conducted by surgical approaches: Case 1 – delayed keratoplasty after remis- antiseptics use. The most effective treatment to combat sion and case 2 – early keratoplasty during a severe flare up of the disease. cysts is chlorhexidine 0.02%, which is prepared ex. In the 1st case the keratitis led to the development of chronic temporae. Polyhexamethylene 0.02% as part of the solu- keratouveitis, secondary glaucoma, complicated cataract, and tion is used for CLs disinfection, and can be used off-label vision loss. The received keratoplasty was of only anatomic in AK. effect. In the 2nd case early keratoplasty allowed avoiding secondary complications and retaining a satisfactory visual Clinical cases provided testify to the difficulties in acuity along with avoiding reoperations. the AK diagnosis and showed features of the disease. Keywords: Acanthamoeba keratitis, Keratitis, Penetrating keratoplasty. CASE REPORTS How to cite this article: Bikbov M, Surkova V, Usubov E. Acanthamoeba Keratitis: Different Surgical Approaches. Int J Case 1 Kerat Ect Cor Dis 2016;5(2):77-80. -

Acanthamoeba Keratitis: First Recorded Case from a Palestinian Patient With
British Journal of Ophthalmology 1996;80:849-853 849 Br J Ophthalmol: first published as 10.1136/bjo.80.9.849 on 1 September 1996. Downloaded from LETTERS TO THE EDITOR Acanthamoeba keratitis: first recorded Table 1 Drug sensitivity ofAcanthamoeba the clinical features in this case were not espe- case from a Palestinian patient with isolatefrom cornea cially reminiscent of those generally recorded trachoma for such an amoebal infection.9 Minimum amoebicidal Traumatic injury is likely to predispose the concentration (fuglml) Keratitis due to Acanthamoeba is a potentially then compromised cornea to Acanthamoeba infection. For the contact lens wearer, there is sight threatening condition if unrecognised, or Drug Trophozoites Cysts if inappropriate medical therapy is used.' The now irrefutable evidence to demonstrate that the protozoa are derived from contaminated infection is being recognised worldwide.'A It Chlorhexidine 1.6 1.6 is often associated in Europe and in the USA Polyhexamethylene tap water,'0 which is used as part of the clean- with contact lens wear; elsewhere, particularly biguanide 3.2 6.3 ing disinfection procedures for the lenses and in the tropics, it occurs most often in rural Alexidine 6.3 6.3 associated paraphernalia. communities and can be associated with Propamidine 6.3 25.0 The observations from this case indicate trauma and mud splashing.5 Pentamidine 12.5 50.0 that chronic trachomatous disease may also Hexamidine 6.3 25.0 compromise the corneal surface such as to We present here the first recorded isolation Neomycin 12.5 50.0 of Acanthamoeba in a Palestinian patient with facilitate invasion by Acanthamoeba, and that keratitis, not associated with either contact this should be considered where other predis- lens wear or the patient's recollection of not used initially because of local unavailabil- posing factors cannot be readily identified. -

Developing Novel Therapeutic Agents for Acanthamoeba Infection and Investigating the Process of Encystment
Developing novel therapeutic agents for Acanthamoeba infection and investigating the process of encystment Anas Abdullah Hamad (BSc, MSc) A thesis submitted in partial fulfilment of the requirements of the University of Wolverhampton for the degree of Doctor of Philosophy June 2020 Declaration This work or any part thereof has not previously been presented in any form to the University or to any other body whether for the purposes of assessment, publication or for any other purpose (unless otherwise indicated in page 3). Save for any express acknowledgements, references and/or bibliographies cited in the work, I confirm that the intellectual content of the work is the result of my own efforts and of no other person. The right of Anas Abdullah Hamad to be identified as author of this work is asserted in accordance with ss.77 and 78 of the Copyright, Designs and Patents Act 1988. At this date copyright is owned by the author. Signature………………………………………. Date……………………………………………. 15/10/2020 2 List of posters and publication related to the work presented in this thesis: Heaselgrave, W., Hamad, A., Coles, S. and Hau, S., 2019. In Vitro Evaluation of the Inhibitory Effect of Topical Ophthalmic Agents on Acanthamoeba Viability. Translational vision science & technology, 8(5), pp.17-17. Manuscript published. Hamad, A. and Heaselgrave, W., 2017. Developing novel treatments for the blinding protozoan eye infection Acanthamoeba keratitis. Proceedings of the Internal Annual Research Symposium, Poster no. 23, University of Wolverhampton, UK. Hamad, A. and Heaselgrave, W., 2018. Developing new treatments and optimising existing treatment strategies for the corneal infection Acanthamoeba keratitis. -

The Immunobiology of <Emphasis Type="Italic">Acanthamoeba
Springer Semin Irnmunopathol (1999) 21 : 147-160 Springer Seminars in Immunopathology © Springer-Verlag 1999 The immunobiology of Acanthamoeba keratitis Jerry Y. Niederkorn, Hassan Alizadeh, Henry F. Leher, James P. McCulley Department of Ophthalmology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75235-9057, USA Background Although only a few centimeters in diameter, the eye is composed of virtually every type of tissue found in the remainder of the body, as well as cellular and noncellular el- ements found nowhere else [37]. The eye is an extension of the brain, and, as such, conducts an enormous array of neurological functions. The million ganglion cells of the retina transmit 500 electrical signals per second, which in computer terms is equiv- alent to 1.5 × ]0 9 bits of information [37]. However, if the cornea loses transparency, complex functions of the retinal elements are rendered meaningless, and acute vision is preempted. To prevent this loss from happening, the eye utilizes anatomical, physi- ological, and immunological barriers to shield the cornea from injury and infection [43]. In many animals the eye is recessed in cranial sockets and surrounded by bony protuberances protecting it from blunt trauma. The eyelids and tear film serve as effective barriers against environmental agents, including air- and water-borne patho- gens. The cornea is exposed continuously to the external environment and potential patho- gens. One might expect any infectious agent encountered at the ocular surface would be met with a vigorous immune response that would promptly eliminate the pathogen and protect the cornea. However, in certain cases, a zealous immune response to corneal pathogens can have the opposite effect, contributing instead to loss of corneal transparency and blindness. -

Randy J. Epstein, MD
Randy J. Epstein, MD BIBLIOGRAPHY 1) PEER-REVIEW JOURNAL ARTICLES 1. Epstein RJ, Hughes WF: “Lymphocyte- induced corneal neovascularization: A morphologic assessment”. Invest Ophthalmol Vis Sci 21 (1):87-94, 1981. 2. Miller MT, Epstein RJ, Sugar J, et. al.: “Anterior segment anomalies associated with the fetal alcohol syndrome”. J Ped Ophthalmol and Strabismus 21:8, 1984. 3. Epstein RJ, Wilson LA, Visvesvara GS, Plourde E: “Rapid diagnosis of Acanthamoeba keratitis from corneal scrapings using indirect fluorescent antibody staining”. Arch Ophthalmol 104:1318, 1986. 4. Epstein RK, Halkias A, Stulting RD, and Rogrigues MM: “Corneal opacities and anterior segment anomalies in DBA/2 mice: Models for corneal elastosis and the iridocorneal endothelial (ICE) syndrome”. Cornea 5:95, 1986. 5. Epstein RJ, Fernandes A, Stulting RD, Tigges M, Wright JD, and Gammon JA: “The correction of aphakia in neonatal monkeys with extended-wear contact lenses: Corneal studies”. Ophthalmology 93: 1495, 1986. 6. Visvesvara GS, Epstein RJ, et al: “Acanthamoeba keratitis associated with contact lenses-United States”. Morbidity and Mortality Weekly Report 35:405, 1988. 7. Epstein RJ, Stulting RD, and Kindle JC: “Blastogenesis induced by Herpes simplex virus in splenic mononuclear cells from inbred mice”. Curr. Eye Res. 6:145, 1987. 8. Epstein RJ, and Stulting RD: “Corneal neovascularization induced by stimulated lymphocytes in inbred mice”. Invest Ophthalmol Vis Sci 28: 1505, 1987. 9. Epstein RJ, Seedor JA, Dreizen NG et al.: “Penetrating keratoplasty for herpes simplex keratitis and keratoconus. Allograft rejection and survival”. Ophthalmology 94: 935, 1987. 10. Epstein RJ, Stulting RD, Hendricks RL, Harris DM: “Corneal neovascularization: Pathogenesis and inhibition”. -

Every Red Eye Deserves an Antibiotic
Ocular Infection Management- The Next Generation Bruce E. Onofrey, OD, RPh, FAAO Professor, U. Houston UEI SERIOUS PROBLEMS REQUIRE SERIOUS DOCUMENTATION THE BIG 6 “I’s” • INFECTION • INFLAMMATION • ISCHEMIA • INJURY • IDIOPATHIC • IATROGENIC (idiotpathic) Just the facts Mrs. Johnson • 65 y/o female visiting her friend Madge from Portland, Oregon. • Madge is one of your favorite long-term patients • Madge refers patient to you • Patient is very concerned about her “eye infection” The Simple Conjunctivitis Case • 65 y/o female recently in LA to visit son • Both developed red eyes • Son told mom he has genital herpes • and chlamydia • Mother seen by local ophthalmologist HX • My vision is getting worse in my left eye • It feels very “sore” and itches a lot • It waters all the time and it feels like my eye is too big for the eye socket • It’s glued shut every morning and very swollen • People are afraid to talk to me –they think I’m going to give them “pink eye” • Am I contagious? Will I lose my vision? Case : cont’d • Mom has Hx of trachoma as child and TB in remission. Worked in a TB ward-Was treated years ago • Mom wears mono-vision CL on OS only. Disposable-wears X wears X 2 weeks. Last worn 9 days ago Case : Cont’d • Eye now very painful and vision very bad • Calif. Dr said the cornea was all “torn up” • The doctor said the drops he gave me would make it better right-away-it made it worse and I stopped it after a day • I’ve been using the new drops daily and taking the pills, but it’s just getting worse every day • Am I going blind?!? QUESTION: Differential DX • 1. -
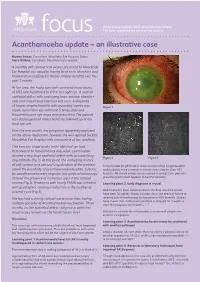
Acanthamoeba Update – an Illustrative Case
An occasional update commissioned by the College. focus The views expressed are those of the authors. Acanthamoeba update – an illustrative case Hamed Anwar, Consultant, Moorfields Eye Hospital, Dubai Mark Wilkins, Consultant, Moorfields Eye Hospital A monthly soft contact lens wearer presented to Moorfields Eye Hospital eye casualty, having been seen elsewhere and treated unsuccessfully for herpes simplex keratitis over the past 1 month. At the time, she had a spectacle corrected visual acuity of 6/12 which pinholed to 6/9 in her right eye. A corneal epithelial defect with underlying haze, anterior chamber cells and conjunctival injection was seen. A diagnosis of herpes simplex keratitis with secondary uveitis was Figure 1 made. Ganciclovir eye ointment 5 times daily and Dexamethasone eye drops were prescribed. The patient was discharged and instructed to be followed up at her local eye unit. Over the next month, her symptoms apparently improved on the above medication, however she was referred back to Moorfields Eye Hospital with a recurrence of her condition. This time the visual acuity in the affected eye had deteriorated to hand motions only, while examination showed a very large epithelial defect with an underlying Figure 2 Figure 3 ring infiltrate (Fig 1). At this point, the underlying history of soft contact lens use and long duration of the problem In the United Kingdom AK is more common than fungal keratitis raised the possibility of acanthaemoeba keratitis. Cultures and probably more common in contact lens wearers than HSV for acanthamoeba were negative, but confocal microscopy keratitis. AK should always be considered in contact lens patients showed the presence of numerous cysts in the corneal presenting with what appears to be HSV keratitis.