Market Access Right Product, Right Patient, Tight Price, Tighter Reimbursement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Synthesis and in Vitro Biochemical Evaluation of Oxime Bond-Linked
Synthesis and in vitro biochemical evaluation of oxime bond- linked daunorubicin–GnRH-III conjugates developed for targeted drug delivery Sabine Schuster1,2, Beáta Biri-Kovács1,2, Bálint Szeder3, Viktor Farkas4, László Buday3, Zsuzsanna Szabó5, Gábor Halmos5 and Gábor Mező*1,2 Full Research Paper Open Access Address: Beilstein J. Org. Chem. 2018, 14, 756–771. 1MTA-ELTE Research Group of Peptide Chemistry, Hungarian doi:10.3762/bjoc.14.64 Academy of Sciences, Eötvös L. University, 1117 Budapest, Hungary, 2Institute of Chemistry, Eötvös L. University, 1117 Budapest, Received: 12 January 2018 Hungary, 3Research Centre for Natural Sciences, Institute of Accepted: 15 March 2018 Enzymology, Hungarian Academy of Sciences, 1117 Budapest, Published: 04 April 2018 Hungary, 4MTA-ELTE Protein Modelling Research Group, Hungarian Academy of Sciences, Eötvös L. University, 1117 Budapest, Hungary This article is part of the Thematic Series "Peptide–drug conjugates". and 5Department of Biopharmacy, Faculty of Pharmacy, University of Debrecen, 4032 Debrecen, Hungary Guest Editor: N. Sewald Email: © 2018 Schuster et al.; licensee Beilstein-Institut. Gábor Mező* - [email protected] License and terms: see end of document. * Corresponding author Keywords: cytostatic effect; daunorubicin; drug-targeting; GnRH derivatives; oxime linkage Abstract Gonadotropin releasing hormone-III (GnRH-III), a native isoform of the human GnRH isolated from sea lamprey, specifically binds to GnRH receptors on cancer cells enabling its application as targeting moieties for anticancer drugs. Recently, we reported on the identification of a novel daunorubicin–GnRH-III conjugate (GnRH-III–[4Lys(Bu), 8Lys(Dau=Aoa)] with efficient in vitro and in vivo antitumor activity. To get a deeper insight into the mechanism of action of our lead compound, the cellular uptake was followed by confocal laser scanning microscopy. -

A Study on Recent Implication of Nanotechnology in Drug Delivery Systems
Available online a t www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2015, 7 (7):213-220 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4 A study on recent implication of nanotechnology in drug delivery systems Mahendra Pratap Singh 1, Parjanya Kumar Shukla 1,2* , Amita Verma 2 and Ramesh Patel 1 1Krishnarpit Institute of Pharmacy, Allahabad 2Department of Pharmaceutical Sciences, Faculty of Health Science, Sam Higginbottom Institute of Agriculture, Technology & Sciences, Allahabad, India _____________________________________________________________________________________________ ABSTRACT Efficient drug targeting to diseases by circumventing all the shortcomings of conventional drug delivery systems can be achieved by the significant approach of advances in nanotechnology. Nanotechnology will affect our lives tremendously over the next decade in very different fields, including medicine and pharmaceutical Sciences. Most of the available drugs now are lipophilic in nature and this stands as challenging aspect faced for scientists to formulate and deliver for better efficacy, so nanoparticles, nanosuspension, nanocapsules are used now days to deliver these drugs with greater bioavailability and also have been adopted to improve the solubility of poorly soluble drugs. The use of nanoparticles is an universal formulation approach to increase the therapeutic performance of drugs in any route of administration. This review article describes the preparation methods, physicochemical -

A Gellan-Based Fluid Gel Carrier for Spray Delivery
AGELLAN-BASEDFLUIDGELCARRIERFOR SPRAY DELIVERY by BRITT TER HORST Athesissubmittedto The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Chemical Engineering College of Engineering and Physical Sciences The University of Birmingham May 2019 UNIVERSITYDF BIRMINGHAM University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Abstract Autologous cell transplantation is a promising approach to enhance burn wound re- epithelialisation. It was introduced to clinical practice decades ago with current delivery techniques involving spraying autologous cultured or uncultured cells in low-viscosity sus- pensions. This delivery method is limited since it results in an uneven distribution on the wound bed and cell loss as the liquid is not retained on the skin surface. In this thesis, a sprayable gel that solidifies on the surface of the skin has been developed to circumvent this problem. A gellan-based fluid gel system was developed with flexible viscoelastic properties that can be tuned by a biocompatible polymer concentration and ionic strength to facilitate spray delivery. The material liquefies at high shear during spraying with self-healing properties of the gel causing it to solidify on the receiving sur- face. -

Powders Are Intimate Mixtures of Dry, Finely Divided Drugs And/Or Chemicals That May Be Intended for Internal Or External Use
Powders are intimate mixtures of dry, finely divided drugs and/or chemicals that may be intended for internal or external use. Advantages/Disadvantages flexibility in compounding good chemical stability Ease of administration time-consuming not well-suited to dispense unpleasant tasting, hygroscopic or deliquescent drugs inaccuracy of dose Types Medicated Aerosol Preparation Weighing of ingredients Comminuting Blending or mixing Weighing of individual dose Materials Active ingredients Packaging Types of material used to pack/dispense divided powders Foil plastic bag used for volatile vegetable parchment thin, semiopaque, moisture-resistant white bond without moisture-resistant properties Glassine glazed, transparent, moisture-resistant paper used for volatile to a certain extent Waxed transparent-water-proof paper used for hygroscopic and volatile drugs Seidlitz powder is the name with which is commonly known a medication composed by a mixture of tartaric acid, sodium bicarbonate, and potassium sodium tartrate, used as a mild cathartic by dissolving in water and drinking. After ingestion, the powder combines with gastric juices developing intestinal gases which are somewhat helpful in evacuating the bowels. This medication's name comes from the Seidlitz Saline Springs of Bohemia (now Sedlčany in the Czech Republic), which were rather famous in Europe at the time this medication was first marketed in the late 19th century, even though the foregoing laxative constituents do not represent those of the springs named. Use of Each ingredient – act as acid and base which react in the presence of water to cause effervescence Use of Preparation - mild cathartic/laxative Appearance – white powder Storage – store in air tight containers Granules are prepared agglomerates of powdered materials, may be used per se for the medicinal value of their content or they may be used for pharmaceutical purposes, as in making tablets. -

Glioblastoma Treatments: an Account of Recent Industrial Developments
REVIEW published: 13 September 2018 doi: 10.3389/fphar.2018.00879 Glioblastoma Treatments: An Account of Recent Industrial Developments Edouard Alphandéry 1,2* 1 Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie, UMR 7590 CNRS, Sorbonne Universités, UPMC, University Paris 06, Paris, France, 2 Nanobacterie SARL, Paris, France The different drugs and medical devices, which are commercialized or under industrial development for glioblastoma treatment, are reviewed. Their different modes of action are analyzed with a distinction being made between the effects of radiation, the targeting of specific parts of glioma cells, and immunotherapy. Most of them are still at a too early stage of development to firmly conclude about their efficacy. Optune, which triggers antitumor activity by blocking the mitosis of glioma cells under the application of an alternating electric field, seems to be the only recently developed therapy with some efficacy reported on a large number of GBM patients. The need for early GBM diagnosis is emphasized since it could enable the treatment of GBM tumors of small sizes, possibly easier to eradicate than larger tumors. Ways to improve clinical protocols by strengthening preclinical studies using of a broader range of different animal and tumor models are also underlined. Issues related with efficient drug delivery and crossing of Edited by: blood brain barrier are discussed. Finally societal and economic aspects are described François E. Paris, University of Nantes, France with a presentation of the orphan drug status that can accelerate the development of Reviewed by: GBM therapies, patents protecting various GBM treatments, the different actors tackling Tullio Florio, GBM disease, the cost of GBM treatments, GBM market figures, and a financial analysis Università di Genova, Italy of the different companies involved in the development of GBM therapies. -

Lipid-Polymer Hybrid Nanoparticles As a Next-Generation Drug Delivery
Providence St. Joseph Health Providence St. Joseph Health Digital Commons Articles, Abstracts, and Reports 1-1-2019 Lipid-polymer hybrid nanoparticles as a next- generation drug delivery platform: state of the art, emerging technologies, and perspectives. Anubhab Mukherjee Department of Translational Neurosciences and Neurotherapeutics, John Wayne Cancer Institute, Pacific euN roscience Institute, Providence Saint John's Health Center, Santa Monica, CA Ariana K Waters Department of Translational Neurosciences and Neurotherapeutics, John Wayne Cancer Institute, Pacific euN roscience Institute, Providence Saint John's Health Center, Santa Monica, CA Pranav Kalyan Achal Singh Achrol Department of Neurosurgery and Neurosciences, John Wayne Cancer Institute and Pacific euN roscience Institute, Santa Monica, CA, USA Santosh Kesari Department of Translational Neuro-Oncology and Neurotherapeutics, John Wayne Cancer Institute at Providence Saint John's Health Center FSeoe nelloxtw pa thige fors aaddndition addal aitutionhorsal works at: https://digitalcommons.psjhealth.org/publications Part of the Oncology Commons Recommended Citation Mukherjee, Anubhab; Waters, Ariana K; Kalyan, Pranav; Achrol, Achal Singh; Kesari, Santosh; and Yenugonda, Venkata, "Lipid- polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives." (2019). Articles, Abstracts, and Reports. 1504. https://digitalcommons.psjhealth.org/publications/1504 This Article is brought to you for free and open access by Providence St. Joseph Health Digital Commons. It has been accepted for inclusion in Articles, Abstracts, and Reports by an authorized administrator of Providence St. Joseph Health Digital Commons. For more information, please contact [email protected]. Authors Anubhab Mukherjee, Ariana K Waters, Pranav Kalyan, Achal Singh Achrol, Santosh Kesari, and Venkata Yenugonda This article is available at Providence St. -

AHRQ Healthcare Horizon Scanning System – Status Update Horizon
AHRQ Healthcare Horizon Scanning System – Status Update Horizon Scanning Status Update: April 2015 Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA290-2010-00006-C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 April 2015 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290-2010-00006-C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. A novel intervention may not appear in this report simply because the System has not yet detected it. The list of novel interventions in the Horizon Scanning Status Update Report will change over time as new information is collected. This should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual target technology reports are developed for those that appear to be closer to diffusion into practice in the United States. A representative from AHRQ served as a Contracting Officer’s Technical Representative and provided input during the implementation of the horizon scanning system. AHRQ did not directly participate in the horizon scanning, assessing the leads or topics, or provide opinions regarding potential impact of interventions. -

Apoptotic Effects of Drug Targeting Conjugates Containing Different Gnrh Analogs on Colon Carcinoma Cells
International Journal of Molecular Sciences Article Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells Eszter Lajkó 1,Rózsa Hegedüs 2,Gábor Mez˝o 2,3 and László K˝ohidai 1,* 1 Department Genetics, Cell- and Immunobiology, Semmelweis University, Nagyvárad tér 4, 1089 Budapest, Hungary; [email protected] 2 Research Group of Peptide Chemistry, Hungarian Academy of Sciences, Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary; [email protected] (R.H.); [email protected] (G.M.) 3 Eötvös Loránd University, Faculty of Science, Institute of Chemistry, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary * Correspondence: [email protected]; Tel.: +36-1-210-2930/56232 Received: 27 July 2019; Accepted: 5 September 2019; Published: 8 September 2019 Abstract: The wide range of cellular target reactions (e.g., antitumor) of gonadotropin-releasing hormone (GnRH) variants provides the possibility to develop multifunctional GnRH conjugates. The aim of our work was to compare the cytotoxic/apoptotic activity of different GnRH-based, daunorubicin (Dau)-linked conjugates with or without butyrated Lys in position 4 (4Lys(Bu)) at a molecular level in a human colorectal carcinoma cell line. Cell viability was measured by impedimetry, cellular uptake and apoptosis were studied by flow cytometry, and the expression of apoptosis-related genes was analyzed by qRT-PCR. The modification with 4Lys(Bu) resulted in an increased cytotoxic and apoptotic effects and cellular uptake of the GnRH-I and GnRH-III conjugates. Depending on the GnRH isoform and the presence of 4Lys(Bu), the conjugates could regulate the expression of several apoptosis-related genes, especially tumor necrosis factor (TNF), tumor protein p53 (TP53) and the members of growth-factor signaling. -
![1 SUPPLEMENTARY DATA Draft Medline Search – Pubmed Interface 1# "Puberty"[Mesh] OR (Puberties) OR (Pubertal Maturati](https://docslib.b-cdn.net/cover/5679/1-supplementary-data-draft-medline-search-pubmed-interface-1-puberty-mesh-or-puberties-or-pubertal-maturati-1835679.webp)
1 SUPPLEMENTARY DATA Draft Medline Search – Pubmed Interface 1# "Puberty"[Mesh] OR (Puberties) OR (Pubertal Maturati
SUPPLEMENTARY DATA Draft Medline search – PubMed interface 1# "Puberty"[Mesh] OR (Puberties) OR (Pubertal Maturation) OR (Early Puberty) OR "Puberty, Precocious"[Mesh] OR (Precocious Puberty) OR "Precocious Puberty, Central" [Supplementary Concept] OR (Central Precocious Puberty) OR "Sexual precocity" [Supplementary Concept] OR (Idiopathic sexual precocity) OR (Familial precocious puberty) OR (Sexual Precocity) OR "Sexual Maturation"[Mesh] OR (Maturation, Sexual) OR (Maturation, Sex) OR (Sex Maturation) OR "Sexual Development"[Mesh] OR (Development, Sexual) OR (Sex Development) OR (Development, Sex) OR (Gonadal Disorder Maturation) OR (Pubertal Onset) OR "Menstruation Disturbances"[Mesh] OR (Precocious Menses) OR (Early Menses) OR (Early Menarche) OR (Precocious Menarche) OR (Disorders of Puberty) OR (Gonadotropin-Dependent Precocious Puberty) OR (Gonadotropin-Independent Precocious Puberty) OR (Isolated Precocious Thelarche) OR (Isolated Precocious Pubarche) OR (Isolated Precocious Menarche) 2# "Gonadotropin-Releasing Hormone"[Mesh] OR (Gonadotropin Releasing Hormone) OR (Luteinizing Hormone-Releasing Hormone) OR (Luteinizing Hormone Releasing Hormone) OR (GnRH) OR (Gonadoliberin) OR (Gonadorelin) OR (LFRH) OR (LH-FSH Releasing Hormone) OR (LH FSH Releasing Hormone) OR (LH-Releasing Hormone) OR (LH Releasing Hormone) OR (LH-RH) OR (LHFSH Releasing Hormone) OR (Releasing Hormone, LHFSH) OR (LHFSHRH) OR (LHRH) OR (Luliberin) OR (FSH-Releasing Hormone) OR (FSH Releasing Hormone) OR (Gn-RH) OR (Factrel) OR (Gonadorelin Acetate) OR (Kryptocur) -
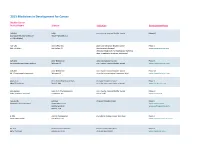
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor -

Nanomaterials and Nanotechnology
nanomaterials and nanotechnology Collection of Selected Papers | 2013 | issn 1847-9804 © Vasil Vasilev/Shutterstock © Vasil Editor-in-Chief Paola Prete Institute for Microelectronics and Microsystems, National Research Council, Lecce, Italy Editorial Board C. N. R. Rao Fellow of the Royal Society, National Research Professor, Linus Pauling Research Professor and President of Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, India Toshiaki Enoki Tokyo Institute of Technology, Japan Stephen O’Brien Department of Chemistry, The City College of New York, USA Juan Ramon Morante Catalonia Institute for Energy Research and University of Barcelona, Spain Stephen Pearton Department of Material Science and Engineering, University of Florida, USA Wolfgang Richter University of Rome Tor Vergata, Italy and Technischen Universität Berlin, Germany Federico Rosei Institut National de la Recherche Scientifique, Universite du Quebec, Varennes, Canada Jonathan E. Spanier Department of Materials Science and Engineering, Drexel University, Philadelphia, USA Leander Tapfer Technical Unit of Materials Technologies Brindisi, ENEA, Italy Reshef Tenne Department of Materials and Interfaces, Weizmann Institute of Science, Rehovot, Israel Fabrice Vallee CNRS and Université Claude Bernard Lyon, France Nanomater. nanotechnol., 2013, Collection of Selected Papers free online editions of InTech Books and Journals can be found at www.intechopen.com Nanomaterials and Nanotechnology Collection of Selected Papers, 2013 Abstracted/Indexed in IET Inspec, Ulrich’s Periodical Directory, Scirus, EBSCO - A-to-Z, WorldCat, BASE - Bielefeld Academic Search Engine, DOAJ - Directory of Open Access Journals, Electronic Journals Library, Google Scholar, CAS - Chemical Abstracts Service, Hrcak Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Identification Statement Online ISSN 1847-9804 Abbreviated key title: Nanomater.