An Investigation of Fusible Tin Boiler Plugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction to Steam Generating Unit (Boiler)
Introduction to Steam Generating Unit (Boiler) A boiler is a closed vessel in which steam is produced from water by combustion of fuel. How a Boiler works:: Water is pumped into the boiler at operating pressure Heat of flue gases vaporizes water to form steam Steam formed is passed into steam space above the water space Applications of steam generatorss (Boilerss)) The steam generators or boilers are integral components of steam turbines which are used as prime movers to drive generators to produce electricity in all thermal and nuclear power plants. These are used in the industry for example in heating systems or for cement production, textiles. These are used to produce distilled water for medicines, pharmaceuticals and other usage. The boilers are used in cold countries for heating large buildings, in agriculture as well for soil steaming. Components of a steam generator (Boiler) Boiler drum, tubes, furnace Boiler mountings Boiler Accessories Boiler mountings are devices which are required for proper operation, safety and control of the boiler. Examples ar ee water level indicator (WLI), pressure gauges (PGs), steam stop valve, safety valves, fusible plug, feed-check valve, blow-off cock, manhole and mudhole, etc. Boiler accessories are devices which are used to increase the efficiency of a boiler. Examples are air preheater, economizer , ss, uperheater , feedpump or injector, baffles, etc.. Boiler classification: 1.1. Tube content: (i) Fire tube boiler and (ii) water tube boiler 2.2. Axis of shell: (i) Horizontal, (ii) vertical, (iii) inclined 3.3. Location of furnace: (i) Externally fired, (ii) internally fired 4.4. -

Steamboating Guide Edition 2 2010
Steamboating SampleGuide Pages Second Edition Steamboating Guide Edition 2 2010 Edited by Roger Calvert and Rob van Es The contributors and editors of this publication have made every effort to ensure the accuracy and relevance of the data presented and the validity and appropriateness of the recommendations made. It is, however, ultimately the responsibility of the owner of a boat to check the data and take the final decisions, in the context of the proposed design. If necessary, appropriate professional advice should be sought. Neither the contributors, the editors, nor the SBA can accept responsibility for any direct or indirect consequences arising from the use of the data or from following the recommendationsSample of this publication. Pages Copying of parts or the whole of this document by members of the SBA is permitted, subject to the terms published on the SBA web site. Otherwise, copying is not permitted without the permission of the SBA, except as allowed under copyright law. Table of Contents Preface Section A – Introduction 1 Hulls 1-1 2 Boiler Types 2-1 3 Engine Types 3-1 4 Fuels 4-1 Section B – Steamboat Operations 5 Boiler Fittings 5-1 6 Steam Plant Installation 6-1 7 Boiler Operation and Maintenance 7-1 8 Steam Ancillaries 8-1 9 Boat Handling Advice 9-1 10 Boiler Inspection and Testing 10-1 11 Trailers and Towing 11-1 Section C – Technical 12 Propulsion 12-1 13 Valve Setting 13-1 14 Data and Performance 14-1 15 Boiler Design Considerations 15-1 16 Workshop Techniques 16-1 Glossary 17-1 Index 18-1 Sample Pages Preface The aims and objects of the Steam Boat Association are: (i) To foster and encourage steam boating and the building, development, preservation and restoration of steam boats and steam machinery, by all possible means. -
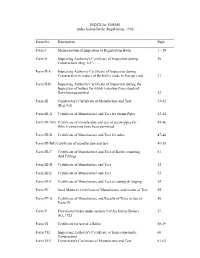
INDEX for FORMS Under Indian Boiler Regulations, 1950
INDEX for FORMS under Indian Boiler Regulations, 1950 ___________________________________________________________________________ Form No. Description Page ___________________________________________________________________________ Form I Memorandum of Inspection or Registration Book 1 - 29 Form II Inspecting Authority's Certificate of Inspection during 30 Construction (Reg. 4-C)…… Form II-A Inspecting Authority Certificate of Inspection during Construction in respect of the boiler made to foreign code 31 Form II-B Inspecting Authority Certificate of Inspection during the Inspection of boilers for which variation from standard Have been permitted 32 Form III Constructor's Certificate of Manufacture and Test 33-42 (Reg.4-d) Form III-A Certificate of Manufacture and Test for Steam Pipes 43-44 Form III-A(I) Certificate of manufacture and test of steam pipes for 45-46 Which variations have been permitted Form III-B Certificate of Manufacture and Test for tubes 47-48 Form III-B(I) Certificate of manufacture and test 49-50 Form III-C Certificate of Manufacture and Test of Boiler mounting 51 And Fittings. Form III-D Certificate of Manufacture and Test 52 Form III-E Certificate of Manufacture and Test 53 Form III-F Certificate of Manufacture and Test of casting & forging 54 Form IV Steel Marker's Certificate of Manufacture and results of Test 55 Form IV-A Certificate of Manufacture and Results of Tests in lieu of 56 Form IV. Form V Provisional Order under section 9 of the Indian Boilers 57 Act, 1923 Form VI Certificate for use of a Boiler 58-59 -

Steam Power Plant
www.getmyuni.com Steam Power Plant A power plant is assembly of systems or subsystems to generate electricity, i.e., power with economy and requirements. The power plant itself must be useful economically and environmental friendly to the society. The present book is oriented to conventional as well as non-conventional energy generation. While the stress is on energy efficient system regards conventional power systems viz., to increase the system conversion efficiency the supreme goal is to develop, design, and manufacturer the non-conventional power generating systems in coming decades preferably after 2050 AD which are conducive to society as well as having feasible energy conversion efficiency and non-friendly to pollution, keeping in view the pollution act. The subject as a whole can be also stated as modern power plants for power viz electricity generation in 21st century. The word modern means pertaining to time. At present due to energy crisis the first goal is to conserve energy for future while the second step is todevelop alternative energy systems including direct energy conversion devices, with the devotion, dedication and determination remembering the phrase, “Delve and Delve Again till wade into”. CLASSIFICATION OF POWER PLANTS Power Plant 1. Conventional - Steam Engines Power Plants - S team Turbine Power Plants - Diesel Power Plants - Gas Turbine Power Plants - Hydro-Electric Power Plants - Nuclear Power Plants Thermoelectric Generator 2. Non-conventional Thermoelectric generator Fuel-cells Power Plants Photovoltaic solar cells Power S ystem MHD Power Plants Fussion Reactor N PP Power S y stem Biogas, Biomass Energy Power sy stem Geothermal Energy Wind Energy Power System Ocean Thermal energy conversion (OTEC) Wave and Tidal Wave Energ y Plantation Scheme A power plant may be defined as a machine or assembly of equipment that generates and delivers a flow of mechanical or electrical energy. -

Guide to Injectors
Advanced Guide to Injectors A little bit here on injectors may be helpful. These are small units that put water into the boiler by using boiler steam against boiler pressure. These are magic, but can often be temperamental and certainly many will not work with warm water. To start, turn on cold water until it flows out of the injector overflow, then turn on the steam slowly and the flow of water should stop. If at first they do not pick up, then shut off the steam and start the process again. Some injectors work better at higher pressures and some at lower, (The size of the internal steam cone makes a difference). You have to get to know which pressure range your injector works best at. To make the injector work you need a good flow of cold water, it is essential that there are no leaks on the suction side as this will draw in air and stop the injector working. NEVER use anything to poke in the steam cones to clean them. Always use a liquid solvent to do this job. Some people even immerse them in coke a cola to clean them and that certainly works, More on Water Levels A word about the hatching may help those that do not know. If a piece of white card or indeed anything painted white is placed behind the gauge glass that has black lines at a 45 degree angle across all the way up it. With this it will show the exact water level very easily. If water is present in the glass when viewed from the front, the lines will show up as straight not angled. -

Locomotive Fireboxes
LOCOMOTIVEFIREBOXES. Paper read before the Institution by Mr. SMITH MANNERING, Member, Brighton, December 9th, 1916. DISCUSSION. Mr. W. A. Lelean: \Ye haxe all listened with the greatest interest to this very practical paper. It will be a most useful contribution to our proceedings. The paper contains such a number of matters for discussion that I am anxious not to take up any of the time of the meeting at the outset and at once, therefore, invite any remarks or criticisms. Mr. R. P. C. Sanderson: Gentlemen,-I would like first to have heard from the English railway men before saying anything myself, because the paper deals largely with cop- per boxes, but in the sixth paragraph of Mr. Mannering’s very interesting paper on firebox construction and main- tenance, he refers to fusible plugs and apparently is desirous of learning other people’s experience. Perhaps I may be able to give some information of interest from my past experience in railway service in the United States. Years ago it was almost universal practice to use fusible plugs in all locomotive crown sheets. Several cases of burned and dropped crown sheets occurred with plain evidence of “ no water,” where the fusible plug was found still intact (un- melted) in the sheet. In the course of the following in- vestigations it was ascertained that :- The new plugs melted at the right temperature and quickly. The old plugs were reasonably clean, the records showing that they had been periodically removed, cleaned and replaced. The melting point of the metal in the old plugs was very high (as I recall it a little above a dull red heat in some cases). -

PART 52—POWER BOILERS 52.20–1 General (Modifies PFT–1 Through PFT–49)
Coast Guard, Dept. of Homeland Security Pt. 52 are required, unless specifically ex- 52.01–35 Auxiliary, donkey, fired thermal empted by other regulations in this fluid heater, and heating boilers. subchapter. 52.01–40 Materials and workmanship. (b) The first inspection of Class II 52.01–50 Fusible plugs (modifies A–19 through A–21). welded pressure vessels shall be per- 52.01–55 Increase in maximum allowable formed during the welding of the longi- working pressure. tudinal joint. At this time the marine 52.01–90 Materials (modifies PG–5 through inspector shall check the material and PG–13). fit-up of the work, and ascertain that 52.01–95 Design (modifies PG–16 through only welders who have passed the re- PG–31 and PG–100). 52.01–100 Openings and compensation (modi- quired tests are employed. fies PG–32 through PG–39, PG–42 through (c) A second inspection of Class II PG–55). welded pressure vessels shall be made 52.01–105 Piping, valves and fittings (modi- during the welding of the circumferen- fies PG–58 and PG–59). tial joints. At this time the marine in- 52.01–110 Water-level indicators, water col- spector shall check any new material umns, gauge-glass connections, gauge being used which may not have been cocks, and pressure gauges (modifies PG– examined at the time of the first in- 60). 52.01–115 Feedwater supply (modifies PG– spection, also the fit-up of the vessel at 61). this stage of fabrication, and in addi- 52.01–120 Safety valves and safety relief tion, observe the welding and ascertain valves (modifies PG–67 through PG–73). -

The Steam Boilers
UNEDITED The Steam Boilers Act being Chapter 177 of The Revised Statutes of Saskatchewan, 1920 (assented to November 10, 1920). FOR HISTORICAL REFERENCE ONLY FOR HISTORICAL NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. Table of Contents SHORT TITLE PART IIII 1 Short title Operation of Boilers INTERPRETATION PROHIBITIONS AND REQUIREMENTS 2 Interpretation 29 Unsafe boilers not used __________ 30 Regulation of pressure on connected boilers 31 Operating without inspection certificate PART I 32 Reduction in working pressure Registration and License 33 Fusible plug 3 Registration of boilers 34 Lock pop safety valve 4 Dealer’s license 5 Nonresident privileges REPORT OF ACCIDENTS 6 License not transferable 35 Owner to report accidents 7 Suspension or cancellation of license ENGINEERS’ AND FIREMEN’S CERTIFICATES 8 Effect of license 36 Examinations 38 Final certificates __________ 39 Qualifications for first class examinations REFERENCE ONLY FOR HISTORICAL PART II 40 Qualifications for second class examination Inspection 41 Qualification for third class examination INSPECTORS 42 Qualification for traction engineer’s examination 9 Appointment 43 Foreign certificate 10 Qualifications 44 Qualifications for provisional certificate -

HGR-B9021-Is01-Inner Firebox Repair and Renewal
Ref No: HGR-B9021 Issue No: 01 Issue Date: April 2012 HERITAGE RAILWAY ASSOCIATION GUIDANCE NOTE INNER FIREBOX REPAIR and RENEWAL Purpose This document describes good practice in relation to its subject to be followed by Heritage Railways, Tramways and similar bodies to whom this document applies. Endorsement This document has been developed with and is fully endorsed by Her Majesty’s Railway Inspectorate, a directorate of the Office of Rail Regulation (ORR). Disclaimer The Heritage Railway Association has used its best endeavours to ensure that the content of this document is accurate, complete and suitable for its stated purpose. However it makes no warranties, express or implied, that compliance with the contents of this document shall be sufficient to ensure safe systems of work or operation. Accordingly the Heritage Railway Association will not be liable for its content or any subsequent use to which this document may be put. Supply This document is published by the Heritage Railway Association (HRA). Copies are available electronically via its website www.heritagerailways.com Issue 01 page 1 of 11 © Heritage Railway Association 2012 The Heritage Railway Association, Limited by Guarantee, is Registered in England and Wales No. 2226245 Registered office: 2 Littlestone Road, New Romney, Kent, TN28 8PL HGR-B9021-Is01 _______________________ Inner Firebox Repair and Renewal Users of this Guidance Note should check the HRA website to ensure that they have the latest version. Table of Contents SECTION Page Number 1. Introduction ........................................................................................................................................... -

07 Fusible Plugs
Please note that this Content may change. These boiler training modules, incorporating sections of the HRA/ORR boiler code of practice, were prepared in 2013 as part of the HLF funded BESTT training plan project and will be progressively reviewed and updated by the BESTT Technical Committee. Fusible Plugs Proposed Syllabus 2013 To be used in conjunction with: HRA Guidance Note HGR-B9008- Is01 BESTT acknowledges the support of the HRA for allowing the use of the Guidance Notes ISSUE 2018 ! Proposed BESTT Syllabus Assessment Plan ! !"#$%&'()*+,,(-../( ( 0$123%&(4%$51( ,621(7"#$%&(21(8"($1&#(29(:"9;$9:82"9(<286(=>?(@$2#A9:&(16&&8()+-../(B( C1.D( ! ?27! "#$%!&'$(!)$**!+$,-!(#-!*-./'-/%!.'!&'0-/%(.'0$'+!12!(#-!2&'3($1'!12!2&%$4*-!5*&+%! &%-0!$'!41$*-/%6! ! C98E"#$:82"9! ! "#$%!&'$(!)$**!+$,-!5/.3($3.*!7'1)*-0+-!128! 96 :'%5-3($1'! ;6 <.(-/$.*%! =6 ".4&*.($1'!12!%$>-%! ?6 "#/-.0!21/@! A6 B-C*-.0$'+! D6 E-.*$'+! F6 "-%($'+! ( ( F&AE9295(G$8:"7&1( "#-!'&@4-/%!$'!5./-'(#-%$%!/-2-/%!(1!(#-!GBH!I&$0.'3-!'1(-!%-3($1'6!! J-./'$'+!1&(31@-!9!31&*0!4-!0-*$,-/-0!$'!.!3*.%%/11@!-',$/1'@-'(6!! ! FGD( 96 K'1)!(#1/1&+#*L!(#-!&%-!12!31//-3(!MMN!21/!(#-!(.%7%!O=P! ;6 K'1)*-0+-!12!@.(-/$.*%!OAP! =6 Q'0-/%(.'0$'+!#1)!.!2&%$4*-!5*&+!2&'3($1'%!OAP! ?6 "L5-%!12!2&%$4*-!5*&+%!OAP! A6 <.$'(-'.'3-!5*.'!OFP! D6 ".4&*.($1'!12!2&%$4*-!5*&+!%$>-%!ORP! F6 Q%-!12!M"SN!4.%-0!31@51&'0%!O9?P! ( FGH( 96 K'1)!(#-!@.(-/$.*%!&%-0!OTP! ;6 K'1)!(#-!()1!(L5-%!12!5*&+!&%-0!ORP! =6 U-!.)./-!12!#1)!(#-!(#/-.0%!31&*0!4-!3&(!O9VP! ?6 Q'0-/%(.'0!*-'+(#!12!5*&+%!O99P! A6 W&(!%1&/3$'+!12!5*&+%!O9;P! -

GEETANJALI INSTITUE of TECHNICAL STUDIES a Practical
GEETANJALI INSTITUE OF TECHNICAL STUDIES (Affiliated to Rajasthan Technical University Kota, Rajasthan) A Practical Training Report On Types of Boilers & Mounting In Mechanical Engineering Session 2012 From 12june-11 july Training at HINDUSTAN ZINC LTD., DEBARI Submitted To: Submitted By: Mechanical Department Bhupendra Singh Jhala Geetanjali Inst. Of Technical Studies B-Tech IV Year Dabok Udaipur VII Semester ACKNOWLEDGEMENT I wish to acknowledge the encouragement received from Mr A.Raman( Principal Of G.I.T.S., Udaipur) ,Mr Vishnu Agarwal (HOD Mech. Department) and special thanks to Mr. Vijendra Sankhala and Mr. Pankaj Ahir for initiating my interest in training. Their mastery & work helped me in covering out this work smoothly. I am also grateful to all the faculty of mechanical departments who helped me to improve my thinking as well as the practical knowledge Bhupendra Singh Jhala CONTENTS 1. Introduction 2. Preface 3. Classification Of Boilers 4. Types Of Boilers 5. Boilers Mounting And Accessories INTRODUCTION Boiler is a metal container in which a liquid is heated and changed into a vapor. Most boilers change water into the vapor steam. Steam is used to heat buildings and processes. It changes from vapor to liquid form as it delivers heat into a room or building, giving off even more heat as a result. Some heating systems, called hydronic systems, circulate hot water rather than steam. However, the heat source in these systems is still referred to as a boiler. Steam produced in boilers is also used in steam turbines and for refining oil or drying paper.The process of heating a liquid until it reaches it's gaseous state is called evaporation Heat is transferred from one body to another by means of (1) radiation, which is the transfer of heat from a hot body to a cold body through a conveying medium without physical contact, (2) convection, the transfer of heat by a conveying medium, such as air or water and (3) conduction, transfer of heat by actual physical contact, molecule to molecule. -

Introduction to Steam Generating Unit (Boiler)
Introduction to Steam Generating Unit (Boiler) A boiler is a closed vessel in which steam is produced from water by combustion of fuel. How a Boiler works: Water is pumped into the boiler at operating pressure Heat of flue gases vaporizes water to form steam Steam formed is passed into steam space above the water space Applications of steam generators (Boilers) The steam generators or boilers are integral components of steam turbines which are used as prime movers to drive generators to produce electricity in all thermal and nuclear power plants. These are used in the industry for example in heating systems or for cement production, textiles. These are used to produce distilled water for medicines, pharmaceuticals and other usage. The boilers are used in cold countries for heating large buildings, in agriculture as well for soil steaming. Components of a steam generator (Boiler) Boiler drum, tubes, furnace Boiler mountings Boiler Accessories Boiler mountings are devices which are required for proper operation, safety and control of the boiler. Examples are water level indicator (WLI), pressure gauges (PGs), steam stop valve, safety valves, fusible plug, feed-check valve, blow-off cock, manhole and mudhole, etc. Boiler accessories are devices which are used to increase the efficiency of a boiler. Examples are air preheater, economizer, superheater, feedpump or injector, baffles, etc. Boiler classification: 1. Tube content: (i) Fire tube boiler and (ii) water tube boiler 2. Axis of shell: (i) Horizontal, (ii) vertical, (iii) inclined 3. Location of furnace: (i) Externally fired, (ii) internally fired 4. Method of circulation: (i) Natural, (ii) forced 5.