Group of Fungicides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

US EPA, Pesticide Product Label, COPPER SULFATE
c C EPA Reg. Number: Date of U.S. ENVIRONMENTAL PROTECTION AGENCY Issuance: Office of Chemical Safety and Pollution Prevention Office of Pesticide Programs 88633-3 AUG 26 20H Registration Division (7504P) 1200 Pennsylvania Ave.', N.W. Washington, DC 20460 Term of Issuance: Conditional NOTICE OF PESTICIDE: X_ Registration Name of Pesticide Product: Reregistration Copper Sulfate [under FIFRA, as amended) Pentahydrate Name and Address of Registrant (include ZIP Code): Mailed to: Delta Agro Chemicals Connie Welch Road 6 No 15 A Agent of Delta Agro Chemicals Maddi, Cairo, Egypt toXcel Toxicology & Regulatory Affairs 7140 Heritage Village Plaza Gainesville, VA 20155 On the basis of information furnished by the registrant, the above named pesticide is hereby registered under the Federal Insecticide, Fungicide and Rodenticide Act Registration is in no way to be construed as an endorsement or recommendation of this product by the Agency. Jn order to protect health and the environment, the Administrator, on his motion, may at any time suspend or cancel the registration of a pesticide in accordance with the Act. The acceptance of any name in connection with the registration of a product under this Act is not to be construed as giving the registrant a right to exclusive use of the name or to its use if it has been covered by others. The basic formulation CSF dated April 9, 2014 of the product referred to above, submitted in connection with registration under the Federal Insecticide, Fungicide, and Rodenticide Act is acceptable. The basic CSF will be added to your file. This product is conditionally registered in accordance with FIFRA section 3(c)(7)(A) provided that you: Page 1 of 2 Signature of Approving Official: Date: AUG 2 6 20H Tony Kish, Product Manager1^) Fungicide Branch/Registration Division/OPP/OCSPP (7504P) EPA Form 8570-6 C~ C" Notice of Pesticide Registration— v_- ""y Copper Sulfate Pentahydrate " // EPA Reg. -

Bordeauxmixture00cran.Pdf
Colleqe 7 Aqrieultwer < icAris University of Illinois Library at Urbana-Champaign ACES ^*2^ UNIVERSITY LIBRARY UNIVERSITY OF ILLINOIS AT URBANA-CHAMPAIGN The person charging this material is responsible for its renewal or return to the library on or before the due r immUm fee for a lost ''em is i>JUu.OO$300 onfn T H $125.00, for bound journals. Theft, and mutilation, underlining of books are reasons or action d.sc.phnary and may result in dismissal he from University. Please note: self-stick notes result in torn may pages and lift some inks. n Via the Tele hone 2f o*o P Center at 217-333-8400 846-262-15-10 (toll-free) or [email protected]. '.uiuc.edu/catalog/ UNIVERSITY OF ILLINOIS Agricultural Experiment Station BULLETIN No. 135 BORDEAUX MIXTURE By CHARLES S. CRANDALL URBANA, ILLINOIS, MAY, 1909 SUMMARY OF BULLETIN No. 135 1. Bordeaux mixture was discovered by accident in the fall of 1882 by Professor Millardet. Page 205 2. Original formulas have been greatly modified. The first formula con- tained more than six times the copper sulphate and nearly 12 times the lime per gallon of water that is used in the present standard 4-4-50 formula. Page 207 3. It is conclusively demonstrated that mixtures made with air-slaked lime are not only extremely injurious to foliage, but are much less adhesive than are mixtures made with fresh-slaked lime. Pages 210 and 288 4. The chemical reactions that occur when copper sulphate and lime are combined take place in a manner to give best results only when the ingredients are combined ki certain definite proportions. -

FRAC Code List ©*2020: Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action (Including FRAC Code Numbering)
FRAC Code List ©*2020: Fungal control agents sorted by cross resistance pattern and mode of action (including FRAC Code numbering) Disclaimer The technical information contained in the global guidelines/the website/the publication/the minutes is provided to CropLife International/RAC members, non-members, the scientific community and a broader public audience. While CropLife International and the RACs make every effort to present accurate and reliable information in the guidelines, CropLife International and the RACs do not guarantee the accuracy, completeness, efficacy, timeliness, or correct sequencing of such information. CropLife International and the RACs assume no responsibility for consequences resulting from the use of their information, or in any respect for the content of such information, including but not limited to errors or omissions, the accuracy or reasonableness of factual or scientific assumptions, studies or conclusions. Inclusion of active ingredients and products on the RAC Code Lists is based on scientific evaluation of their modes of action; it does not provide any kind of testimonial for the use of a product or a judgment on efficacy. CropLife International and the RACs are not responsible for, and expressly disclaim all liability for, damages of any kind arising out of use, reference to, or reliance on information provided in the guidelines. Listing of chemical classes or modes of action in any of the CropLife International/RAC recommendations must not be interpreted as approval for use of a compound in a given country. Prior to implementation, each user must determine the current registration status in the country of use and strictly adhere to the uses and instructions approved in that country. -

Bordeaux Mixture
BORDEAUX MIXTURE Integrated Pest Management for Backyard Orchardists and Home Gardeners Bordeaux mixture is an outstanding as Bordeaux mix. They are most effec- fungicide and bactericide that has been tive and are a better choice to use in used for decades to control some dis- spring after the trees have broken dor- eases of tree fruits and nuts, vine fruits, mancy and tender leaves are exposed. and ornamentals. The ability of Bor- To be effective for certain pathogens deaux mixture to weather the fall, win- such as the leaf curl fungus, the fixed ter, and spring rains and to adhere to copper compound must contain at least plants makes it an excellent choice for 50% copper. With all Bordeaux and a winter fungicide. If Bordeaux mix- fixed copper sprays, thorough cover- ture is applied in spring after the tree age is essential to give plants the de- breaks dormancy, use weaker, more sired protection from disease-causing dilute formulations of the mixture to pathogens. Advantages and disadvan- reduce the risk of plant injury. The ap- tages of both sprays are outlined in plication of Bordeaux during hot Table 1. weather may cause yellowing and leaf peach and nectarine, downy mildew drop. Leaf burn may occur if rain Bordeaux is also commercially avail- and powdery mildew fungi in grapes, occurs soon after a Bordeaux applica- able in premixed packages; however, peacock spot pathogen in olives, wal- tion. To reduce the chance of leaf burn, these products are not nearly as effec- nut blight bacteria in walnut, and black add 1 quart of spray oil for every 100 tive as freshly made Bordeaux. -

Clinical Epidemiology and Treatment Outcome of Hexaconazole Poisoning – a Prospective Six Year Study
Hexaconazole - Clinical epidemiology and treatment outcome A. Anand & P. Abinash ORIGINAL ARTICLE ORIGINAL ARTICLE Clinical epidemiology and treatment outcome of Hexaconazole poisoning – A prospective six year study ACHARYA ANAND1 and PANDA ABINASH2 1Department of Pharmacology, Konaseema Institute of Medical Sciences & Research Foundation, Amalapuram, Andhra Pradesh, India 2Department of Pharmacology, M.K.C.G. Medical College, Berhampur, Odisha, India. Abstract Background: Hexaconazole is a category 3/4 of poison as per the W.H.O Expert Group on Pesticide Residues. Hexaconazole is used to control infection by fungi in paddy and other crops. Apart from destroying the target species, it can also cause damage to humans. There have been discrete reports of instances of human poisoning due to hexaconazole. Methodology: A patient record-based cross-sectional study was carried out in Konaseema Institute of Medical Science & Research Foundation, Amalapuram, Andhra Pradesh, India during a period from March 2014 to April 2020 on 26 confirmed cases of hexaconazole poisoning. The clinic-demographic data, hematological, and biochemical parameters at the time of admission and at 72 hrs as well as the outcome were recorded and analyzed using descriptive statistics and paired t test. Result: The prevalence of hexaconazole poisoning was 4.79% of all poisoning cases. The major clinical presentation was gastrointestinal symptoms with vomiting being commonest. There was no significant change in the biochemical and hematological parameters. The mean duration of hospitalization was 4.93+1.39 days. The recovery rate was 100% without any major sequel. Conclusion: Poisoning due to hexaconazole is uncommon in comparison to poisoning by other pesticides in the agricultural community. -

Garden Chemicals and Their Application
1 Garden Chemicals and Their Application A fungicide is a toxicant or poison for fungi, a chemical or physical agent that kills or inhibits the development of fungus spores or mycelium. It may be an eradicant, applied to a plant, plant part, or the environment as a curative treatment to destroy fungi established within a given area or plant; or preferably it may be a protectant, applied to protect a plant or plant part from infection by killing, or inhibiting the development of, fungus spores or mycelium that may arrive at the infection court. A bactericide is a toxicant or poison for bacteria. Among the more recent bactericides are antibiotics, products of other living organisms. They also have value against certain fungi. There are also a few virocides, which are toxic or poisonous to viruses. A disinfectant is an agent that frees a plant or plant part from infection by destroying the pathogen established within it. A disinfestant kills or inacti vates organisms present on the surface of the plant or plant part or in the immediate environment. Chemicals for seed treatment can be either eradicants or protectants, but most of them are disinfestants, in that they kill organisms on the surface of the seed rather than those within. In common usage, however, they are called disinfectants. A nematicide is, of course, a chemical that kills nematodes in the soil or in the plant. Most nematicides are fumigants, chemical toxicants that act in volatile form. Not so long ago the chemicals on the garden medicine shelf consisted of copper and sulfur for protectants, lime sulfur as an eradicant, mercuric chloride as a disinfectant, and formalin and carbon bisulfide as fumigants. -
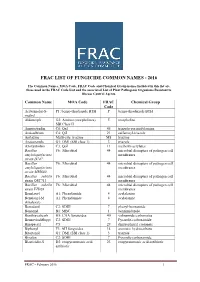
Frac List of Fungicide Common Names - 2016
FRAC LIST OF FUNGICIDE COMMON NAMES - 2016 The Common Names, MOA Code, FRAC Code and Chemical Group names included in this list are those used in the FRAC Code List and the associated List of Plant Pathogenic Organisms Resistant to Disease Control Agents. Common Name MOA Code FRAC Chemical Group Code Acibenzolar-S- P1: benzo-thiadiazole BTH P benzo-thiadiazole BTH methyl Aldimorph G2: Amines (morpholines) 5 morpholine SBI Class II Ametoctradin C8: QxI 45 triazolo-pyrimidylamine Amisulbrom C4: QiI 21 sulfamoyl-triazole Anilazine Multi-site: triazine M8 triazine Azaconazole G1: DMI (SBI class 1) 3 triazole Azoxystrobin C3: QoI 11 methoxy-acrylates Bacillus F6: Microbial 44 microbial disrupters of pathogen cell amyloliquefaciens membranes strain D747 Bacillus F6: Microbial 44 microbial disrupters of pathogen cell amyloliquefaciens membranes strain MBI600 Bacillus subtilis F6: Microbial 44 microbial disrupters of pathogen cell strain QST713 membranes Bacillus subtilis F6: Microbial 44 microbial disrupters of pathogen cell strain FZB24 membranes Benalaxyl A1: Phenylamide 4 acylalanine Benalaxyl-M A1: Phenylamide 4 acylalanine (kiralaxyl) Benodanil C2: SDHI 7 phenyl-benzamide Benomyl B1: MBC 1 benzimidazole Benthiavalicarb H5: CAA fungicides 40 valinamide carbamates Benzovindiflupyr C2: SDHI 7 Pyrazole-carboxamide Binapacryl C5 29 dinitrophenyl crotonate Biphenyl F3: AH fungicides 14 aromatic hydrocarbons Bitertanol G1: DMI (SBI class 1) 3 triazole Bixafen C2: SDHI 7 Pyrazole-carboxamide Blasticidin-S D2: enopyranuronic acid 23 enopyranuronic -

Antifungal Drugs Are Those Drugs Which Inhibit Or Retard Fungal Growth
Prepared by- Dr Arpita Shrivastav DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA Antifungal drugs are those drugs which inhibit or retard fungal growth. DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA Sites of action of antifungal drugs Chitin + Glucan DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA SITE OF ACTION DRUGS Cell membrane Ergosterol binding Amphotericin B, Natamycin and Nystatin Ergosterole synthesis inhibitors Allylamines (Amorolfine, (Squalene epoxidase inhibitor) Terbinafine etc.) Ergosterol synthesis inhibitors Azoles (Ketoconazole, Miconazole (lanosterol to ergosterol) etc.) Cell wall β glucan synthase inhibitor Echinocandins Intracelluler Pyrimidine analogue Flucytosine Mitotic inhibitors Gresiofulvin Other site Benzoic acid , salicylic acid, tolnaftate, castor oil etc. DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA Source – Streptomyces nodosus Mechanism of action – Increases permeability of cell membrane by binding to ergosterol Antimicrobial spectrum – broad spectrum Resistance – when less ergosterol in membrane Pharmacokinetics – poorly absorbed from GIT, do not cross BBB Adverse effects - Nephrotoxicity Drug interaction – 1. with miconazole antagonistic effect 2. with flucytosine synergistic effect Dose – Dog -0.25 to 0.50 mg/kg, 3 times weekly Cat – 0.1 to 0.50 mg/kg, 3 times weekly DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA Source – Streptomyces noursie Streptomyces aureus Mechanism of action – It binds to ergosteol and forms pores in cell membrane DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA Source - Streptomyces natalensis Mechanism of action – Binds to ergosterol and cause leakiness of cell membrane DR ARPITA SHRIVASTAV ASSTT. PROFESSOR VETERINARY COLLEGE REWA Pentameric ring compounds containing at least one other non-carbon atom of either nitrogen, sulfur, or oxygen. -

Objective Plant Pathology
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/305442822 Objective plant pathology Book · July 2013 CITATIONS READS 0 34,711 3 authors: Surendra Nath M. Gurivi Reddy Tamil Nadu Agricultural University Acharya N G Ranga Agricultural University 5 PUBLICATIONS 2 CITATIONS 15 PUBLICATIONS 11 CITATIONS SEE PROFILE SEE PROFILE Prabhukarthikeyan S. R ICAR - National Rice Research Institute, Cuttack 48 PUBLICATIONS 108 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Management of rice diseases View project Identification and characterization of phytoplasma View project All content following this page was uploaded by Surendra Nath on 20 July 2016. The user has requested enhancement of the downloaded file. Objective Plant Pathology (A competitive examination guide)- As per Indian examination pattern M. Gurivi Reddy, M.Sc. (Plant Pathology), TNAU, Coimbatore S.R. Prabhukarthikeyan, M.Sc (Plant Pathology), TNAU, Coimbatore R. Surendranath, M. Sc (Horticulture), TNAU, Coimbatore INDIA A.E. Publications No. 10. Sundaram Street-1, P.N.Pudur, Coimbatore-641003 2013 First Edition: 2013 © Reserved with authors, 2013 ISBN: 978-81972-22-9 Price: Rs. 120/- PREFACE The so called book Objective Plant Pathology is compiled by collecting and digesting the pertinent information published in various books and review papers to assist graduate and postgraduate students for various competitive examinations like JRF, NET, ARS conducted by ICAR. It is mainly helpful for students for getting an in-depth knowledge in plant pathology. The book combines the basic concepts and terminology in Mycology, Bacteriology, Virology and other applied aspects. -

(12) United States Patent (10) Patent No.: US 8,110,689 B2 Wada Et Al
US008110689B2 (12) United States Patent (10) Patent No.: US 8,110,689 B2 Wada et al. (45) Date of Patent: *Feb. 7, 2012 (54) BENZANILIDES WITH INSECTICIDAL M. Waksmundzka-Hajnos, “Chromatographic Separation of Nitro ACTIVITY Phenones and Their Reduced Derivatives on Thin Layers of Polar Adsorbents”, XP-008069069, pp. 159-171. Jun-Ichi Inoh et al., “Palladium-Catalyzed Coupling Reaction of (75) Inventors: Katsuaki Wada, Tochigi (JP); Tetsuya 4-Alkylnitrobenzenes with Aryl Bromides at Their Benzylic Posi Murata, Tochigi (JP); Katsuhiko tion”, XP-004130892, Tetrahedron Letters, 1998, pp. 4673-4676. Shibuya, Tochigi (JP); Eiichi Shimojo, M. Makosza et al., “Synthesis of (p-Nitroaryl)diarylmethanes via Vicarious Nucleophilic Substitution of Hydrogen', XP-002399354. Tochigi (JP) Synthesis, No. 9, 2000, pp. 1237-1240. S. Florio et al., “Vicarious Nucleophilic Substitution of (73) Assignee: Bayer Cropsciene AG, Monheim (DE) (Chloroalkyl)heterocycles with Nitroarenes', XP-002399353, Eur, J. Org. Chem., 2004, pp. 2118-2124. (*) Notice: Subject to any disclaimer, the term of this W. Waiers, “Some Substitution Reactions of patent is extended or adjusted under 35 4-Aminodiphenylmethane', XP-008069039, pp. 1060-1064. G. Esselen, Jr., “The Splitting of Benzhydrols by the Action of Bro U.S.C. 154(b) by 403 days. mine”, XP-002399352, pp. 308-324. This patent is Subject to a terminal dis M.Z.A. Badr et al., “Molecular Rearrangements. 14. Photolysis and claimer. Thermolysis of Phenylpropionanilides”, XP-0023993.51, J. Org. Chem., vol. 44, No. 18, 1979, pp. 3244-3247. Y. Watanabe et al., “Stilbene Derivative. Its Preparation, and (21) Appl. No.: 11/917,411 Electrophotographic Photoreceptor Using Same', XP-002399363, 2000, 2 pgs. -

Modern Fungicides and Antifungal Compounds VI
buchcover_fungizides18-8-2011 18.08.2011 9:51 Uhr Seite 1 C M Y CM MY CY CMY K Persistent Identifier: urn:nbn:de:0294-sp-2011-Reinh-8 Spectrum Phytomedizin Spectrum Phytomedizin Proceedings of the 16th International Reinhardsbrunn Symposium on Modern Fungicides and Antifungal Com- pounds, 2010 The Proceedings of the 16th International Reinhardsbrunn Symposium on Modern Fungicides and Antifungal Com- H.W. Dehne, H.B.Gisi, Deising, U. K.Lyr (Eds.) Kuck, P.E. Russell, H. H. H.W. Dehne, H.B. Deising, U. Gisi, K. H. Kuck, P.E. Russell, H. Lyr (Eds.) pounds uphold the tradition of all previous Symposium Proceedings in this series by bringing you research reports on the increasingly complex discipline of fungicide science. As technology develops and new priorities are set for the Modern Fungicides and control of plant diseases, the research undertaken to provide the tools for disease control expands. In order to Antifungal Compounds VI use these tools efficiently, information must be available on the biological and chemical properties of the disease control agents. The present Proceedings provide a unique insight into current research and are an invaluable source of reference for students and established scientists. Modern Fungicides and Antifungal Compounds VI 16th International Reinhardsbrunn Symposium Spectrum Phytomedizin April 25 – 29, 2010 Friedrichroda, Germany ISBN: 978-3-941261-10-5 Probedruck Persistent Identifier: urn:nbn:de:0294-sp-2011-Reinh-8 DPG Spectrum Phytomedizin H. W. DEHNE, H. B. DEISING, U. GISI, K. H. KUCK, P. E. RUSSELL, H. LYR (EDS) Modern Fungicides and Antifungal Compounds VI Proceedings of the 16th International Reinhardsbrunn Symposium April 25 -29, 2010, Friedrichroda, Germany Persistent Identifier: urn:nbn:de:0294-sp-2011-Reinh-8 ISBN: 978-3-941261-10-5 Das Werk einschließlich aller Teile ist urheberrechtlich geschützt. -

Tutorial on Fungicides
Online Guide To GRAPEVINE DISEASES Virginia Tech Tutorial on Fungicides Ashley L. Myers Grape Pathology Extension Specialist, Department of Plant Pathology, Physiology, and Weed Sciences, Virginia Tech AHS Agricultural Research and Extension Center, Winchester, VA Pesticides can be described by their time of “invention”; by their mode of action (MOA), their descriptors which include mobility, function, and time of use, or their chemical class. Often all this becomes a tangled mess and confusion (even among the most knowledgeable) can set in. It’s no small wonder that novel (or even veteran) grape growers would feel a bit overwhelmed. Added to all the names, dates, amounts, preharvest intervals, restricted entry levels, tank mixes, etc, etc, etc is the issue that has us all shaking in our spray boots – resistance development! But how do we know how to delay resistance development if we don’t understand the pesticide chemistries? Topping it all off, how do you pick the chemical with the best bang for the buck without knowledge of the brand names in each chemical class? Whoa…let’s not get ahead of ourselves. In fact, let’s go back to square one and introduce one component of chemical control: the fungicides. What is a fungicide: The definition of a fungicide is a chemical that kills fungi. The cide portion of the word comes from Latin meaning to kill . By contrast certain fungistatic materials may inhibit but not kill fungi. High temperature, for example, can be fungistatic (and may even be fungicidal if high enough). Other terms used to classify fungicides (which I call descriptors) relate to mobility, function, and time of use.