D02 DIGESTIVE DRAINAGE- Aloe, Activated Charcoal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
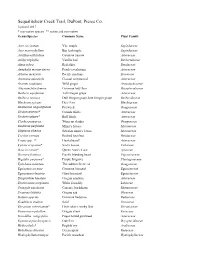
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

City of Vancouver Native Trees and Shrubs Last Revision: 2010 Plant Characteristics (A - M)
City of Vancouver Native Trees and Shrubs Last Revision: 2010 Plant Characteristics (A - M) *This list is representative, but not exhaustive, of the native trees and shrubs historically found in the natural terrestrial habitats of Vancouver, Washington. Botanical Name Common NameGrowth Mature Mature Growth Light / Shade Tolerance Moisture Tolerance Leaf Type Form Height Spread Rate Full Part Full Seasonally Perennially Dry Moist (feet) (feet) Sun Sun Shade Wet Wet Abies grandies grand fir tree 150 40 medium evergreen, 99 999 conifer Acer circinatum vine maple arborescent 25 20 medium deciduous, shrub 99 99 broadleaf Acer macrophyllum bigleaf maple tree 75 60 fast deciduous, 99 999 broadleaf Alnus rubra red alder tree 80 35 very fast deciduous, 99 999 broadleaf Amalanchier alnifolia serviceberry / saskatoon arborescent 15 8 medium deciduous, shrub 99 99 broadleaf Arbutus menziesii Pacific madrone tree 50 50 very slow evergreen, 99 9 broadleaf Arctostaphylos uva-ursi kinnikinnick low creeping 0.5 mat- fast evergreen, shrub forming 999 broadleaf Berberis aquifolium tall Oregon-grape shrub 8 3 medium evergreen, (Mahonia aquilfolium) 99 99 broadleaf Berberis nervosa low Oregon-grape low shrub 2 3 medium evergreen, (Mahonia aquifolium) 99 9 99 broadleaf Cornus nuttalli Pacific flowering dogwood tree 40 20 medium deciduous, 99 99 broadleaf Cornus sericea red-osier dogwood shrub 15 thicket- very fast deciduous, forming 99 9 9 9 broadleaf Corylus cornuta var. californica California hazel / beaked shrub 20 15 fast deciduous, hazelnut 99 9 9 broadleaf -

Plot B510 Plot D305 Close-Up and Overview Photos of PEM1
Plot A641 Plot B510 Plot C308 Plot D305 Close-up and overview photos of PEM1 Vegetation Community during June 2010 Plot C341 Plot D530 Plot E380 East end Transect B Close-up and overview photos of PEM1 vegetation community during June 2016 Plot D87 Plot A554 Plot C51 Plot H69 Close-up and overview photos of PEM2 Vegetation Community during June 2010 Plot A340 Plot D189 Plot E131 Plot E167 Close-up and overview photos of PEM2 vegetation community during June 2016 Plot H89 Plot H101 Plot G67 Plot Close-up and overview photos of PEM3 Vegetation Community during June 2010 Plot B43 Plot C63 Plot H216 Plot H136 Close-up and overview photos of PEM3 vegetation community during June 2016 Plot F138 Plot F118 Plot F138 Close-up and overview photos of PEM4 Vegetation Community During June 2010 Plot I226 Plot B154 Plot I255 Plot I255 Close-up and overview photos of PSS Vegetation Community during June 2010 West End Transect A Plot C568 Plot D364 Plot I150 Close-up and overview photos of PSS vegetation community during June 2016 Plot D625 Plot F64 Plot B644 Plot D567 Close-up and overview photos of Riparian 1 Vegetation Community during June 2010 Plot A640 East End Transect A East End Transect D East End Transect E Close-up and overview photos of Riparian 1 vegetation community during June 2016 Plot G102 Plot F246 Plot I410 Plot I410 Close-up and overview photos of Riparian 2 Vegetation Community during June 2010 East End Transect F Plot F281 Plot I378 Close-up and overview photos of Riparian 2 vegetation community during June 2016 ______________________________________________________________________________ -

Monografia-Rhamnus.Pdf
1 MINISTÉRIO DA SAÚDE MONOGRAFIA DA ESPÉCIE Rhamnus purshiana (CÁSCARA SAGRADA) Organização: Ministério da Saúde e Anvisa Fonte do Recurso: Ação 20K5 (DAF/SCTIE/MS)/2012 Brasília 2014 2 LISTA DE ILUSTRAÇÕES Figura 1 - Aspecto geral da espécie Rhamnus purshiana D.C. 1A: planta; 1B: casca do caule; 1C: folhas e flores; 1D: folhas e frutos 11 Figura 2 - Aspecto geral da droga vegetal (cascas) de Rhamnus purshiana D.C. 14 Figura 3 - Estruturas encontradas no pó da droga vegetal Rhamnus purshiana D.C. I e IA: fibras circundadas por prismas de oxalato de cálcio. 2: esclereides, mostrando um fragmento de camada de cristal (cr.) 3: fragmentos de cortiça e córtex em corte seccional, mostrando grupamento de cristais de oxalato de cálcio. 4: prismas e grupos de cristais de oxalato de cálcio. 5: vista superficial de fragmentos de células corticais. 6: fragmento de musgo. 7. Parte de um raio medular em corte tangencial longitudinal com parênquima contendo pontuações. 8. Floema em corte radial longitudinal mostrando um tubo crivado com placas crivadas (s.p.), parênquima contendo aglomerados de cristais de oxalato de cálcio e raio medular. 9: colênquima do córtex mostrando pontuações (pt.). 10: parênquima contendo grãos de amido. 11: células de floema parenquimatoso, mostrando excrescências na parede. 12: fragmentos de hepáticas. 16 Figura 4 - Principais metabólitos secundários encontrados em Rhamnus purshiana D.C. 28 Figura 5 - Esquema de biossíntese de derivados antracênicos 29 3 LISTA DE TABELAS Tabela 1 - Características da droga vegetal Rhamnus -

Tribe Species Secretory Structure Compounds Organ References Incerteae Sedis Alphitonia Sp. Epidermis, Idioblasts, Cavities
Table S1. List of secretory structures found in Rhamanaceae (excluding the nectaries), showing the compounds and organ of occurrence. Data extracted from the literature and from the present study (species in bold). * The mucilaginous ducts, when present in the leaves, always occur in the collenchyma of the veins, except in Maesopsis, where they also occur in the phloem. Tribe Species Secretory structure Compounds Organ References Epidermis, idioblasts, Alphitonia sp. Mucilage Leaf (blade, petiole) 12, 13 cavities, ducts Epidermis, ducts, Alphitonia excelsa Mucilage, terpenes Flower, leaf (blade) 10, 24 osmophores Glandular leaf-teeth, Flower, leaf (blade, Ceanothus sp. Epidermis, hypodermis, Mucilage, tannins 12, 13, 46, 73 petiole) idioblasts, colleters Ceanothus americanus Idioblasts Mucilage Leaf (blade, petiole), stem 74 Ceanothus buxifolius Epidermis, idioblasts Mucilage, tannins Leaf (blade) 10 Ceanothus caeruleus Idioblasts Tannins Leaf (blade) 10 Incerteae sedis Ceanothus cordulatus Epidermis, idioblasts Mucilage, tannins Leaf (blade) 10 Ceanothus crassifolius Epidermis; hypodermis Mucilage, tannins Leaf (blade) 10, 12 Ceanothus cuneatus Epidermis Mucilage Leaf (blade) 10 Glandular leaf-teeth Ceanothus dentatus Lipids, flavonoids Leaf (blade) (trichomes) 60 Glandular leaf-teeth Ceanothus foliosus Lipids, flavonoids Leaf (blade) (trichomes) 60 Glandular leaf-teeth Ceanothus hearstiorum Lipids, flavonoids Leaf (blade) (trichomes) 60 Ceanothus herbaceus Idioblasts Mucilage Leaf (blade, petiole), stem 74 Glandular leaf-teeth Ceanothus -

Lääkeluettelon Rohdokset , Liite 2. Luonnos Drogerna I
LÄÄKELUETTELON ROHDOKSET , LIITE 2. LUONNOS 1 DROGERNA I LÄKEMEDELSFÖRTECKNINGEN , BILAGA 2. UTKAST Latinankielinen nimi, Suomenkielinen nimi, Ruotsinkielinen nimi, Englanninkielinen nimi, Latinskt namn Finskt namn Svenskt namn Engelskt namn Abri semen (Abrus precatorius) Paternosterpapu Paternosterböna Abrus precatorius Paternosterpapu Paternosterböna Indian Liquorice Absinthii herba (Artemisia absinthium) Koiruoho, Mali Äkta malört Wormwood Acanthopanax senticosus (=Eleutherococcus s.) Venäjänjuuri Ryskrot Acanthopanaxsessilifloru s (=Eleutherococcus s.) Kiinanaralehti Kinesisk stickaralia Aconiti tuber (Aconitum napellus) Ukonhatunmukula Stormhatts knöl Common Monkshood / Mouse Aconitum napellus Aitoukonhattu Stormhatt bane / Frair's Cap Aconitum sp. Ukonhatut Stormhatt Monkshood Calamus / Cinnamon Sedge / Acorus calamus Rohtokalmojuuri Kalmus Sweet Flag Adonidis herba (Adonis vernalis) Adonisyrtti Adonisört Pheasant´s eye Adonis vernalis Kevätruusuleinikki Våradonis Yellow Pheasant's Eye Aegle marmelos Belahedelmä Belafrukt Indian bael Common Horse Chestnut / Aesculus hippocastanum Hevoskastanja Hästkastanj Horse Chestnut / Buckeye Chaste berry / Chaste Tree / Agni casti fructus Hemp Tree / Chastelamb / (Vitex agnus castus) Siveydenpuun hedelmä Kyskhetsträd, Munkpeppar Monk´s pepper Aloe barbadensis Barbados aloes / Mediterranean (=Aloe vera) Lääkeaaloe Såraloe aloes Aloe capensis Kapin aaloe Kap-Aloe Cape aloes Aloe sp. Aaloet Aloe Aloes Aloe vera Barbados aloes / Mediterranean (=Aloe barbadensis) Lääkeaaloe Såraloe aloes Amanita -

CASCARA SAGRADA.Pdf
HERBALPEDIA CASCARA SAGRADA summer, and dried for one to two years before use in decoctions, liquid extracts, powders, and tablets. History: Rhamnus is Latin for buckthorns and purshiana after Fredrick Pursh, a German botanist. Cascara sagrada, a name given by Spanish-Mexicans, means sacred bark dating back to the seventeenth century, when it was bestowed on this tree by Spanish and Mexican explorers. Apparently, they were intrigued by the American Indians use of the bark for a wide Frangula purshiana variety of medicinal purposes. It was first listed [FRANG-yoo-luh pur-shee-AH-nuh] in the US Pharmacopoeia in 1890. It is mild (syn Rhamnus purshiana) enough for use in treating children and the elderly. Indiscriminate stripping of bark, Family: Rhamnaceae leading to the destruction of some 100,000 trees a year, was reported as early as 1909. Names: cascara buckthorn, sacred bark, Honey produced from cascara flowers also has chittem bark, bearwood, bearberry, coffeeberry a slight laxative effect. bark, Mountain cranberry bark, bitter bark, Ecorce Sacree, yellow bark, cttim bark, Persian Constituents: astringent compounds, tannin, bark; Kushina, Joster (Russian); Amerikanische bitter compounds (Anthraquinone glycosides); Faulbaumrinde (German); k!labuq!wacbupt emodin glycosides, together with aloin-like (Makah); tatsa’bats (Skagit); k’ladyats glycosides, cascarosides, chrysaloin, (Squaxin) chrysophanol, aloe-emodin, bitter principle, tannins, ferment and resin Description: Lax evergreen shrub or tree, hardy to 5F with a height of 10-40 feet, spread Properties: A bitter, astringent, cooling herb of 10-30 feet with obovate, irregularly toothed, that has a tonic effect on the liver and digestive deeply veined dull green leaves up to 6 inches system, and acts as a laxative; nervine; emetic. -

Woodard Bay Natural Resources Conservation Area Vascular Plant List
Woodard Bay Natural Resources Conservation Area Vascular Plant List Courtesy of DNR staff and the Washington Native Plant Society. Nomenclature follows Flora of the Pacific Northwest 2nd Edition (2018). * - Introduced Genus/Species Common Name Plant Family Abies grandis Grand fir Pinaceae Acer circinatum Vine maple Sapindaceae Acer macrophyllum Bigleaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Actaea rubra Baneberry Ranunculacae Adenocaulon bicolor Path finder, trail plant Asteraceae Adiantum aleuticum (A. pedantum) Northern maidenhair fern Pteridaceae Agrostis exarata Spike bentgrass Poaceae Aira caryophyllea* Silver hairgrass Poaceae Amelanchier alnifolia Western serviceberry, saskatoon Rosaceae Anaphalis margaritacea Pearly everlasting Asteraceae Anthemis cotula* Stinking chamomile, mayweed chamomile Asteraceae Aquilegia formosa Red columbine Ranunculaceae Arbutus menziesii Pacific madrone Ericaceae Arctostaphylos uva-ursi Kinnikinnick Ericaceae Arctium minus* Common burdock Asteraceae Arum italicum* Italian arum Araceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium filix-femina Common lady fern, northern lady fern Athyriaceae Atriplex patula* Saltbush, spearscale Amaranthaceae Bellis perennis* English lawn daisy Asteraceae Mahonia aquifolium Tall Oregon grape Berberidaceae Mahonia nervosa Dull Oregon-grape, Low Oregon-grape Berberidaceae Struthiopteris spicant Deer fern Blechnaceae Brassica nigra* Black mustard Brassicaceae Bromus spp** Brome grasses Poaceae -

Show Activity
A Purgative *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Achyranthes aspera Chaff Flower 1 Aconitum napellus Garden Wolfsbane; Helmet Flower; Garden Monkshood; Monkshood; Aconite; Blue Rocket; Friar's Cap; Turk's 1 Cap; Bear's-Foot; Soldier's Cap; European Aconite; Queen's Fettle Aconitum carmichaelii Aconite; Fu-Tsu 2 Actaea spicata European Baneberry 1 Actaea rubra Red Baneberry 1 Actaea pachypoda Doll's-Eyes; American Baneberry; White Cohosh; White Baneberry; Baneberry 1 Aesculus hippocastanum Horse Chestnut 1 Alnus glutinosa Black Alder 1 Aloe vera Aloe; Bitter Aloes 7 Althaea officinalis White Mallow; Marshmallow 1 Anastatica hierochuntica Jericho Rose 1 Andira araroba Araroba 1 Anemone pulsatilla Pasque Flower 1 Areca catechu Pin-Lang; Betel Nut 1 2000.0 Artemisia capillaris Capillary Wormwood 1 Boehmeria nivea Ramie 1 Buxus sempervirens Boxwood 1 Camellia sinensis Tea 1 Capsella bursa-pastoris Shepherd's Purse 1 Cassia tora Sickle Senna 5 Cassia marilandica Wild Senna 2 2.0 Cassia grandis Pink Shower 1 Citrullus colocynthis Colocynth 1 Clematis vitalba Traveler's Joy 1 Cocos nucifera Copra; Nariyal; Coconut Palm; Coconut; Kokospalme (Ger.); Cocotero (Sp.) 1 Colchicum autumnale Meadow Saffron; Autumn Crocus 1 24600.0 Convallaria majalis Lily-Of-The-Valley 1 Cornus florida American Dogwood 1 Crataegus rhipidophylla Hawthorn 1 Crataegus laevigata Whitethorn; Hawthorn; English Hawthorn; Woodland Hawthorn 1 Croton tiglium Purging Croton 1 Daphne genkwa Yuan Hua 1 Equisetum arvense Horsetail; Field Horsetail 1 Eremurus chinensis 1 Euphrasia officinalis Eyebright 1 Fallopia japonica Japanese Knotweed; Hu-Zhang; Giant Knotweed; Mexican Bamboo 2 13400.0 Frangula purshiana Cascara Buckthorn; Cascara Sagrada 6 Frangula alnus Buckthorn 6 Dr. -

Elk River Natural Resources Conservation Area Vascular Plant List
Elk River Natural Resources Conservation Area Vascular Plant List Courtesy of DNR staff. Nomenclature follows Flora of the Pacific Northwest 2nd Edition (2018). Scientific Name Common Name Family Achillea millefolium yarrow ASTERACEAE Agrostis stolonifera * red top GRAMINEAE Alnus rubra red alder BETULACEAE Angelica lucida sea-watch APIACEAE Atriplex prostrata * fat hen CHENOPODIACEAE Calamagrostis nutkaensis Pacific reedgrass POACEAE Carex lyngbyei Lyngby’s sedge CYPERACEAE Carex obnupta slough sedge CYPERACEAE Cuscuta salina salt-marsh dodder CONVOLVULACEAE Deschampsia caespitosa tufted hairgrass POACEAE Distichlis spicata saltgrass POACEAE Festuca rubra red fescue POACEAE Frangula purshiana cascara RHAMNACEAE Gaultheria shallon salal ERICACEAE Glaux maritima saltwort PRIMULACEAE Grindelia integrifolia gumweed ASTERACEAE Isolepis cernua low clubrush CYPERACEAE Jaumea carnosa jaumea ASTERACEAE Juncus balticus Baltic rush JUNCACEAE Lilaeopsis occidentalis lilaeopsis APIACEAE Lonicera involucrata black twinberry CAPRIFOLIACEAE Malus fusca western crabapple ROSACEAE Picea sitchensis Sitka spruce PINACEAE Plantago maritima seaside plantain PLANTAGINACEAE Polystichum munitum western swordfern DRYOPTERIDACEAE Potentilla anserina ssp. pacifica Pacific silverweed ROSACEAE Pseudotsuga menziesii Douglas-fir PINACEAE Pteridium aquilinum western brackenfern DENNSTAEDTIACEAE Rubus spectabilis salmonberry ROSACEAE Rubus ursinus trailing blackberry ROSACEAE Rumex occidentalis western dock POLYGONACEAE Salicornia depressa pickleweed AMARANTHACEAE Sidalcea -

Herb Profile
HerbalGram 120 • Oct HerbalGram — Dec 2018 Ayahuasca Field Report • ABC’s 30th Anniversary & Timeline • Sustainable Herbs Program Indian Kino Tree Reforestation • Industry B Corps • Coca-Cola Acquires Moxie • Herb Profile: Senna Senna Profile • ABC’s 30th Anniversary & Timeline • Sustainable Herbs Program • Indian Kino Tree Reforestation • Industry B Corps • Coca-Cola Acquires Moxie • Industry Acquires Reforestation • Coca-Cola B Corps Tree • Indian Kino • Sustainable Herbs Program Timeline 30th Anniversary & • ABC’s Senna Profile The Journal of the American Botanical Council Number 120 Oct – Dec 2018 Senna Herb Profile www.herbalgram.org US/CAN $6.95 30th Anniversary Issue American Botanical Council Mark Blumenthal Founder, Executive Director HerbalGram Editor-in-Chief dear reader Hannah Bauman On November 1, 1988, I went to the Secretary of State’s office HerbalGram Associate Editor in Austin, Texas, to file the nonprofit incorporation papers for the Toby Bernal American Botanical Council. The initial Board of Trustees comprised Head Gardener eminent economic botanist James A. Duke, PhD, internationally Janie Carter esteemed pharmacognosist Professor Norman R. Farnsworth, PhD, Membership Coordinator and me. (Professor Varro “Tip” E. Tyler, PhD, became the fourth Caroline Caswell Trustee after he retired from Purdue University the following year.) Education Assistant The primary motivation for founding ABC was to create a nonprofit Jackson Curtin vehicle to enhance the publication of HerbalGram, which, at the time, Communications & Marketing Coordinator was a small quarterly newsletter that I had published for five years with Rob McCaleb under the auspices of the American Herbal Products Association and the Herb Gayle Engels Simplicity Special Projects Director Research Foundation that Rob founded. -

Taraxacum Officinale, Berberis Vulgaris, Rhamnus Purshiana
COLOSODE - taraxacum officinale, berberis vulgaris, rhamnus purshiana, rheum, cina, arsenicum album, aloe socotrina, colostrum, gallbladder, hepar bovinum, liquid Apotheca Company Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective. ---------- Colosode ACTIVE INGREDIENTS: Taraxacum officinale 1X, Berberis vulgaris 3X, Rhamnus purshiana 3X, Rheum 3X, Cina 3X, 6X, 12X, Arsenicum album 6X, Aloe socotrina 6X, 12X, 30X, Colostrum 8X, Gallbladder 8X, 12X, Hepar bovinum 8X, 12X, Carbo vegetabilis 8X, 30X, Intestine 8X, 30X, Aranea diadema 12X, Carbo animalis 12X, Enterococcinum 12X, Mandragora officinarum 12X, Mercurius solubilis 12X, Selenium metallicum 12X, Veratrum album 12X, Lactobacillus 15X, Clostridium botulinum 30X, 60X, Proteus mirabilis 30X, 60X, Salmonella enteritidis 30X, 60X, Pseudomonas aeruginosa 30X, 60X, Yersinia enterocolitica 30X, 60X, Indolum 30X, 60X, Clostridium perfringens 15C, 30C, 60C, Escherchia coli 32C, Giardia lamblia 12C, 30C, 60C, 200C. INDICATIONS: Aids in the relief of acute food poisoning, and removal of bowel toxin residue. WARNINGS: Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. If pregnant or breast-feeding, ask a health professional before use. Do not use if tamper evident seal is broken or missing. DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age. INACTIVE INGREDIENTS: Demineralized water, 25% Ethanol. KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away. INDICATIONS: Aids in the relief of acute food poisoning, and removal of bowel toxin residue.