Molluscan Distribution Patterns in Fanning Island Lagoon and a Comparison of the Mollusks of the Lagoon and the Seaward Reefsi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jfifo G>Nco Pttsftst
The malacologicalsocietymalacological society ofJapanof Japan 51 HJ2ts j?III71J v -f' Lxi a 7 -V JFiF o G>nc o pttsftst - i7-7VB- o. kIV 7 riVV- }; Remarks on the Taxonomy of Japanese Nassariidae Walter O. CERNOHORSKY (Auckland Institute and Museum, Auckland, New Zealand) Text-figs. 1-19) (fimp of a revision The examination of type-specirnens Nassariidae in preparation for of Indo-Pacific Nassariidae, has brought to light new information which has a bearing on the established taxonorny of Nassariidae, in the way of chronelogical priority and alterations in synonymy. In case of Japanese Nassariidae, a DuNKER species is introduced into Japanese literature, while the synonyrny of other species is elucidated. Nassariidae Family Nassariidae IREDALE, 1916 Genus IVassarius DuMERIL, 1806 Subgenus Hima LEAcH in GRAy, 1852 Hima LEAcH in GRAy, 1852, Moll. Britan. Synop., p. 123, Type-species by sub- minutum PENNANT, 1777 sequent designation(MARwlcK, 1931) Buccinum =B. incrassatum STRoM, 1768. 1839 in Syn. 1852 Tritonella A. ADAMs, Proc. Zool. Soc. Lond. p. 111 (non SwAiNsoN, Amphibia). 1936 Reticunassa IKEDALE, Rec. Aust. Mus., 19 (5), p, 322. Type species by original designation Aikessa Patipera GouLD, 1850. The author has pointed out the taxonomic aspects of Hima and Reticunassa in an earlier paper (CERNOHORSKy, 1972). No constant generic or subgeneric character could be found which whould clearly separate Aima from Reticunassa. IVassarius (Hima) pamperus (GOULD, 1850) VJ E・ uiA:;/p ffi (Text-figs. 7-12) 1850 IVkssa Pampera GouLD, Proc. Bost. Soc. Nat. HisL, 3, p. 155; 1852 GouLD, U. S. Expl. ne. Exp., 12, p. 262, pL 19, figs. -

Animal Spot Animal Spot Uses Intriguing Specimens from Cincinnati Museum Center’S Collections to Teach Children How Each Animal Is Unique to Its Environment
Animal Spot Animal Spot uses intriguing specimens from Cincinnati Museum Center’s collections to teach children how each animal is unique to its environment. Touch a cast of an elephant’s skull, feel a real dinosaur fossil, finish a three-layer fish puzzle, observe live fish and use interactives to explore how animals move, “dress” and eat. Case 1: Modes of Balance and Movement (Case design: horse legs in boots) Animals walk, run, jump, fly, and/or slither to their destination. Animals use many different parts of their bodies to help them move. The animals in this case are: • Blue Jay (Cyanocitta cristata) • Grasshopper (Shistocerca americana) • Locust (Dissosteira carolina) • Broad-wing damselfly (Family: Calopterygidae) • King Rail (Rallus elegans) • Eastern Mole (Scalopus aquaticus) • Brown trout (Salmo trutta) • Gila monster (Heloderma suspectum) • Damselfly (Agriocnemis pygmaea) • Pufferfish (Family: Tetraodontidae) • Bullfrog (Rona catesbrana) • Cicada (Family: Cicadidae) • Moths and Butterflies (Order: Lepidoptera) • Sea slugs (Order: Chepalaspidea) • Koala (Phascolarctos cinereus) • Fox Squirrel (Sciurus niger) • Giant Millipede (Subspecies: Lules) Case 2: Endo/Exoskeleton (Case design: Surrounded by bones) There are many different kinds of skeletons; some inside the body and others outside. The animals with skeletons on the inside have endoskeletons. Those animals that have skeletons on the outside have exoskeletons. Endoskeletons • Hellbender salamander (Genus: Cryptobranchus) • Python (Family: Boidae) • Perch (Genus: Perca) -

Prof. Henry Ramos Matthews” of the Instituto De Ciências Do Mar, Universidade Federal Do Ceará
MOLLUCAN TYPES IN THE MALACOLOGICAL COLLECTION “PROF. HENRY RAMOS MATTHEWS” OF THE INSTITUTO DE CIÊNCIAS DO MAR, UNIVERSIDADE FEDERAL DO CEARÁ Arquivos de Ciências do Mar Espécimes-tipos de moluscos da Coleção Malacologica professor “Henry Ramos Matthews” do Instituto de Ciências do Mar, Universidade Federal do Ceará Cristina de Almeida Rocha-Barreira1, Helena Matthews-Cascon2, Luzymeire da Silva Souza1 ABSTRACT The molluscan types incorporated during the last 50 years in the Malacological Collection “Prof. Henry Ramos Matthews” were inventoried and the original descriptions of each species presented. This Collection presents 18 types, representing 11 gastropods species and one scaphopod species: Metula anfractura Matthews & Rios, 1968; Mitra saldanha Matthews & Rios, 1970; Mitra lopesi Matthews & Coelho, 1969; Ancilla faustoi Matthews et al., 1977; Caducifer atlanticus Coelho et al., 1970; Bursa barcellosi Matthews et al., 1973; Bursa pacamoni Matthews & Coelho,1971; Bursa natalensis Coelho & Matthews,1970; Malea noronhensis Kempf & Matthews, 1969; Marginella cloveri Matthews & Rios, 1972; Latirus lacteum Matthews-Cascon et al, 1991; and Dentalium elegantulum Penna- Neme, 1974. Most of the molluscan taxa are from the North and Northeast of Brazil and were described by Dr. Henry Ramos Matthews and his colleagues. Key words: Malacological Collection “Prof. Henry Ramos Matthews’’, name-bearing types, Brazil. RESUMO Os espécimes-tipo de moluscos incorporados ao longo dos últimos 50 anos na Coleção Malacológica “Prof. Henry Ramos Matthews” -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean. -

The Ultrastructure of Spermatozoa and Spermiogenesis in Pyramidellid Gastropods, and Its Systematic Importance John M
HELGOLANDER MEERESUNTERSUCHUNGEN Helgol~inder Meeresunters. 42,303-318 (1988) The ultrastructure of spermatozoa and spermiogenesis in pyramidellid gastropods, and its systematic importance John M. Healy School of Biological Sciences (Zoology, A08), University of Sydney; 2006, New South Wales, Australia ABSTRACT: Ultrastructural observations on spermiogenesis and spermatozoa of selected pyramidellid gastropods (species of Turbonilla, ~gulina, Cingufina and Hinemoa) are presented. During spermatid development, the condensing nucleus becomes initially anterio-posteriorly com- pressed or sometimes cup-shaped. Concurrently, the acrosomal complex attaches to an electron- dense layer at the presumptive anterior pole of the nucleus, while at the opposite (posterior) pole of the nucleus a shallow invagination is formed to accommodate the centriolar derivative. Midpiece formation begins soon after these events have taken place, and involves the following processes: (1) the wrapping of individual mitochondria around the axoneme/coarse fibre complex; (2) later internal metamorphosis resulting in replacement of cristae by paracrystalline layers which envelope the matrix material; and (3) formation of a glycogen-filled helix within the mitochondrial derivative (via a secondary wrapping of mitochondria). Advanced stages of nuclear condensation {elongation, transformation of fibres into lamellae, subsequent compaction) and midpiece formation proceed within a microtubular sheath ('manchette'). Pyramidellid spermatozoa consist of an acrosomal complex (round -

Mediterranean Triton Charonia Lampas Lampas (Gastropoda: Caenogastropoda): Report on Captive Breeding
ISSN: 0001-5113 ACTA ADRIAT., ORIGINAL SCIENTIFIC PAPER AADRAY 57(2): 263 - 272, 2016 Mediterranean triton Charonia lampas lampas (Gastropoda: Caenogastropoda): report on captive breeding Mauro CAVALLARO*1, Enrico NAVARRA2, Annalisa DANZÉ2, Giuseppa DANZÈ2, Daniele MUSCOLINO1 and Filippo GIARRATANA1 1Department of Veterinary Sciences, University of Messina, Polo Universitario dell’Annunziata, 98168 Messina, Italy 2Associazione KURMA, via Andria 8, c/o Acquario Comunale di Messina-CESPOM, 98123 Messina, Italy *Corresponding author: [email protected] Two females and a male triton of Charonia lampas lampas (Linnaeus, 1758) were collected from March 2010 to September 2012 in S. Raineri peninsula in Messina, (Sicily, Italy). They were reared in a tank at the Aquarium of Messina. Mussels, starfish, and holothurians were provided as feed for the tritons. Spawning occurred in November 2012, lasted for 15 days, yielding a total number of 500 egg capsules, with approximately 2.0-3.0 x 103 eggs/capsule. The snail did not eat during the month, in which spawned. Spawning behaviour and larval development of the triton was described. Key words: Charonia lampas lampas, Gastropod, triton, veliger, reproduction INTRODUCTION in the Western in the Eastern Mediterranean with probable co-occurrence in Malta (BEU, 1985, The triton Charonia seguenzae (ARADAS & 1987, 2010). BENOIT, 1870), in the past reported as Charonia The Gastropod Charonia lampas lampas variegata (CLENCH AND TURNER, 1957) or Cha- (Linnaeus, 1758) is a large Mediterranean Sea ronia tritonis variegata (BEU, 1970), was recently and Eastern Atlantic carnivorous mollusk from classified as a separate species present only in the Ranellidae family, Tonnoidea superfamily, the Eastern Mediterranean Sea (BEU, 2010). -

Mollusca:Gastropoda) in the New World Emily H
THE GENUS HARPA (MOLLUSCA:GASTROPODA) IN THE NEW WORLD EMILY H. VOKES TULANE UNIVERSITY I. ABSTRACT the eastern Atlantic (H. doris Roding - The name Harpa americana has been H. rosea Lamarck) and one in the eastern applied to every fossil Harpa specimen Pacific (H. crenata Swainson). Two fossil found in the New World. A second exam species come from the Miocene of Europe, ple of true H. americana from the Gurabo and of course, the two Caribbean and east Formation, Dominican Republic, shows ern Pacific forms mentioned above as the that none should be so referred and the New World fossil record.* form that occurs in the Agueguexquite The only described species of Harpa in the Caribbean is that one originally refer Formation of Mexico is here named H. is red by Gabb (1873, p. 214) to the West Afri thmica, n. sp. The examples taken from can "H. rosea" and subsequently renamed the Esmeraldas Formation of Ecuador are H. americana by Pilsbry (1922, p. 337). better referred to the living West Coast Known only to come from the Dominican species H. crenata. Republic, with neither exact locality nor stratigraphic level being certain, and II. INTRODUCTION based upon a single incomplete shell, H. The gastropod genus Harpa is a good ex americana for some time remained the ample of the group Woodring termed only name consi,dered for any example of "paciphiles," that is, present in the Ter Harpa in the Neogene of the Caribbean. tiary of the Caribbean but now extinct Thus, when Perrilliat Montoya (1960) there while still living on the eastern monographed the "Middle Miocene" fauna Pacific coast (see Woodring, 1966, p. -
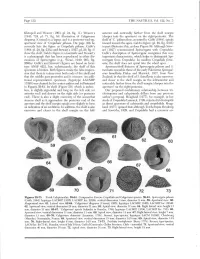
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave. -

Wamsi Node 4 Milestone Progress Report
WAMSI 3.2.2b Intertidal Invertebrates 1. Executive Summary 2. Key Findings and Recommendations 4. Communication and Outputs 30 May 2011 Diversity, abundance and distribution of intertidal invertebrate species in the Ningaloo Marine Park Final Report 30 May 2011 WAMSI Project 3.2.2b 1 of 30 WAMSI 3.2.2b Intertidal Invertebrates 1. Executive Summary 2. Key Findings and Recommendations 4. Communication and Outputs 30 May 2011 1. Executive Summary 1.1 Date 30 May 2011 1.2 Project Title & Number 3.2.2b Diversity, abundance and distribution of intertidal invertebrate species in the NMP 1.3 Project Leader Dr. Robert Black, School of Animal Biology, University of Western Australia 1.4 Project Team Prof. Michael S. Johnson, School of Animal Biology, University of Western Australia; Dr. Jane Prince, School of Animal Biology and Oceans Institute, UWA; Dr. Anne Brearley, School of Plant Biology and Oceans Institute, UWA; 1.5 Dates Covered July 2007 to May 2011 1. 6 Summary (for even shorter account see section 2.1) Aims and approach A quantitative pilot study of the composition of the benthic community of macro- invertebrates on intertidal rocky platforms was undertaken to (A) provide detailed information on variation in biodiversity along the length of the Ningaloo Marine Park and (B) determine the appropriate design of a monitoring protocol powerful enough to determine predefined levels of change. These general overall aims were in the context of the Ningaloo Marine Park Draft Management Plan of 2004, which set out a vision of maintaining the ecological values in the Park, and protecting it from adverse human impacts. -

Mollusca, Gastropoda
Contr. Tert. Quatern. Geol. 32(4) 97-132 43 figs Leiden, December 1995 An outline of cassoidean phylogeny (Mollusca, Gastropoda) Frank Riedel Berlin, Germany Riedel, Frank. An outline of cassoidean phylogeny (Mollusca, Gastropoda). — Contr. Tert. Quatern. Geo!., 32(4): 97-132, 43 figs. Leiden, December 1995. The phylogeny of cassoidean gastropods is reviewed, incorporating most of the biological and palaeontological data from the literature. Several characters have been checked personally and some new data are presented and included in the cladistic analysis. The Laubierinioidea, Calyptraeoidea and Capuloidea are used as outgroups. Twenty-three apomorphies are discussed and used to define cassoid relations at the subfamily level. A classification is presented in which only three families are recognised. The Ranellidae contains the subfamilies Bursinae, Cymatiinae and Ranellinae. The Pisanianurinae is removed from the Ranellidae and attributed to the Laubierinioidea.The Cassidae include the Cassinae, Oocorythinae, Phaliinae and Tonninae. The Ranellinae and Oocorythinae are and considered the of their families. The third the both paraphyletic taxa are to represent stem-groups family, Personidae, cannot be subdivided and for anatomical evolved from Cretaceous into subfamilies reasons probably the same Early gastropod ancestor as the Ranellidae. have from Ranellidae the Late Cretaceous. The Cassidae (Oocorythinae) appears to branched off the (Ranellinae) during The first significant radiation of the Ranellidae/Cassidaebranch took place in the Eocene. The Tonninae represents the youngest branch of the phylogenetic tree. Key words — Neomesogastropoda, Cassoidea, ecology, morphology, fossil evidence, systematics. Dr F. Riedei, Freie Universitat Berlin, Institut fiir Palaontologie, MalteserstraBe 74-100, Haus D, D-12249 Berlin, Germany. Contents superfamily, some of them presenting a complete classifi- cation. -

Effects of Anthropogenic Disturbances of Tropical Soft-Bottom Benthic Communities
MARINE ECOLOGY PROGRESS SERIES Published March 17 Mar Ecol Prog Ser l Effects of anthropogenic disturbances of tropical soft-bottom benthic communities Patrick Frouin* Laboratoire d'ecologie marine, UniversitB de la Reunion. BP 7151,97715 Saint Denis Messag Cedex 9. France ABSTRACT: The benthic ecosystem of the lagoon surrounding Tahiti, the most populated island of French Polynesia, was investigated to assess the impacts of terrestrial runoff on these benthlc cornmu- nities. Five lagoonal zones based on population densities around the coast of Tahti were identified, and within each zone a transect from the fringing reef to the barrier reef was sampled, a total of 18 stations. Only large macrofauna collected on a 2 mm sieve were considered in this study. Multivariate analysis uslng total biomass and environmental factors showed that the stations formed 3 main groups which were related to sediment characteristics, including percentage of silt, organic matter and phaeop~g- ment levels. The distribution of the major feeding groups was related to the amounts of terrestrial inputs and distance from the shore. The stations on the barrier reef and those in zones adjacent to low population areas were not impacted by these terrestrial inputs. Deposit-feeding communities of capitel- lid polychaetes were dominant in the channel parts of the lagoon, which acted as decanting ponds. Chaetopterid polychaetes played an important role in recycling sediments of terrigenous origin in the fringing ecosystem. The patterns of diversity, density and biomass of the benthos around the lagoon revealed that some areas were impacted by moderate terrigenous inputs. It appears that the interme- diate disturbance hypothesis explains the functioning of the parts of the benthic lagoonal ecosystem which are subjected to human impact. -

Caenogastropoda
13 Caenogastropoda Winston F. Ponder, Donald J. Colgan, John M. Healy, Alexander Nützel, Luiz R. L. Simone, and Ellen E. Strong Caenogastropods comprise about 60% of living Many caenogastropods are well-known gastropod species and include a large number marine snails and include the Littorinidae (peri- of ecologically and commercially important winkles), Cypraeidae (cowries), Cerithiidae (creep- marine families. They have undergone an ers), Calyptraeidae (slipper limpets), Tonnidae extraordinary adaptive radiation, resulting in (tuns), Cassidae (helmet shells), Ranellidae (tri- considerable morphological, ecological, physi- tons), Strombidae (strombs), Naticidae (moon ological, and behavioral diversity. There is a snails), Muricidae (rock shells, oyster drills, etc.), wide array of often convergent shell morpholo- Volutidae (balers, etc.), Mitridae (miters), Buccin- gies (Figure 13.1), with the typically coiled shell idae (whelks), Terebridae (augers), and Conidae being tall-spired to globose or fl attened, with (cones). There are also well-known freshwater some uncoiled or limpet-like and others with families such as the Viviparidae, Thiaridae, and the shells reduced or, rarely, lost. There are Hydrobiidae and a few terrestrial groups, nota- also considerable modifi cations to the head- bly the Cyclophoroidea. foot and mantle through the group (Figure 13.2) Although there are no reliable estimates and major dietary specializations. It is our aim of named species, living caenogastropods are in this chapter to review the phylogeny of this one of the most diverse metazoan clades. Most group, with emphasis on the areas of expertise families are marine, and many (e.g., Strombidae, of the authors. Cypraeidae, Ovulidae, Cerithiopsidae, Triphori- The fi rst records of undisputed caenogastro- dae, Olividae, Mitridae, Costellariidae, Tereb- pods are from the middle and upper Paleozoic, ridae, Turridae, Conidae) have large numbers and there were signifi cant radiations during the of tropical taxa.