Lawrence Berkeley National Laboratory Recent Work
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chair's Message Making Chemical Testing Relevant to Breast Cancer
Newsletter February 2011 Santa Clara Valley Section American Chemical Society Volume 33 No. 2 FEBRUARY 2011 NEWSLETTER TOPICS March Dinner Meeting • March Dinner Meeting: Making Making Chemical Testing Chemical Testing Relevant to Breast Cancer Relevant to Breast Cancer • Chair’s Message Dr. Megan R. Schwarzman • February Dinner Meeting: Sex, Love Abstract ductive environmental health, and Oxytocin Although breast cancer is U.S. and European chemicals one of the leading causes of can- policy, and the implications for • Welcome to the Santa Clara Valley cer and death in women, even human health and the environ- Section of ACS the small numbers of chemicals ment of the production, use and • New Members List for December that undergo safety testing are disposal of chemicals and prod- • Volunteer with Kids and Chemistry not routinely evaluated for their ucts. She is a research scientist at impacts on mammary (breast) the Center for Occupational and • Black History Month tissue. Likewise, there is no well- Environmental Health (COEH), • Committee Chair Needed established set of tests for screen- in UC Berkeley’s School of Public • Albert Ghiorso, Nuclear Researcher ing chemicals for their ability to raise the risk Health, and Associate Director of Health and • SPLASH is Coming to Stanford of breast cancer. Environment for the interdisciplinary In 2010, Dr. Schwarzman served as continued on next page Principal Investigator of a project to tackle this Chair's Message issue. The Breast Cancer and Chemicals Policy project, supported by a grant from the March Dinner Meeting As I write this, California Breast Cancer Research Program, Joint Meeting with Palo Alto AWIS we’ve just experi- convened a panel of 20 scientists and policy enced a New Year experts to review the biological mechanisms Date: Wednesday, March 23, 2011 celebration and associated with breast cancer and propose a Time: 7:00 Networking Dinner jumped into the strategy for screening and identifying chemi- 7:30-7:45 Announcements International Year of cals that could increase the risk of the disease. -

Albert Ghiorso 1915-2010
Albert Ghiorso 1915-2010 Albert Ghiorso, the celebrated nuclear scientist who co-discovered more elements than anyone in history and a prolific inventor of nuclear techniques and machines, passed away from natural causes on 26 December 2010 in Berkeley, California. Ghiorso was born in Vallejo, California, on 15 July 1915. He became fascinated with airplanes and aeronautics when he moved with his family to Alameda, near the Oakland airport. That exposure triggered his lifelong interest in machines, technology, and electronics. After high school he began experimenting with radio, and soon his projects were exceeding even military capabilities. On a scholarship, he attended the University of California, Berkeley, and in 1937 he received a BS in electrical engineering. His first job, with a firm developing Geiger counters, brought him in contact with the nuclear chemistry group at Berkeley. That led to an invitation from Glenn Seaborg to join him at the University of Chicago’s Metallurgical Laboratory, which was responsible for elaborating the chemistry of plutonium as part of the Manhattan Project. Thus began their lifetime collaboration, which eventually resulted in the addition of 12 new elements to the periodic table and numerous innovations in nuclear chemistry, radiation detection, and accelerator science. Ghiorso’s first work in Chicago involved measuring the energies of alpha particles from isotopes produced by irradiation of uranium, neptunium, and plutonium. Ghiorso and colleagues built an electromechanical 48-channel pulse-height analyzer, which they used to spectroscopically resolve the characteristic nuclear radiation, thus providing the first nonchemical means of isotope identification. In 1944–45 the Seaborg group used the reactor at the Clinton Laboratories (later Oak Ridge National Laboratory) and cyclotrons at Washington University in Saint Louis and at Berkeley to irradiate plutonium-239 with neutrons and deuterons. -

Peaceful Berkelium
in your element Peaceful berkelium The first new element produced after the Second World War has led a rather peaceful life since entering the period table — until it became the target of those producing superheavy elements, as Andreas Trabesinger describes. f nomenclature were transitive, element 97 with the finding that in Bk(iii) compounds would be named after an Irish bishop spin–orbit coupling leads to a mixing of the Iwhose philosophical belief was that first excited state and the ground state. This material things do not exist. But between gives rise to unexpected electronic properties the immaterialist George Berkeley and the not present in analogous lanthanide element berkelium stands, of course, the structures containing terbium. Even more fine Californian city of Berkeley (pictured), recently5, a combined experimental and where the element was first produced in computational study revealed that berkelium December 1949. The city became the eponym can have stabilized +iii and +iV oxidation of element 97 “in a manner similar to that states also under mild aqueous conditions, used in naming its chemical homologue indicating a path to separating it from other terbium […] whose name was derived from lanthanides and actinides. the town of Ytterby, Sweden, where the rare The main use of berkelium, however — earth minerals were first found”1. arguably one that wouldn’t have met with A great honour for the city? The mayor Bishop Berkeley’s approval — remains a of Berkeley at the time reportedly displayed PHOTO STOCK / ALAMY AF ARCHIVE distinctly material one: as the target for “a complete lack of interest when he was the synthesis of other transuranium and called with the glad tidings”2. -

History of the Seaborg Institute
HISTORY OF THE SEABORG INSTITUTE ARQ.2_09.cover.indd 1 6/22/09 9:24 AM ContentsContents History of the Seaborg Institute The Seaborg Institute for Transactinium Science Established to educate the next generation of nuclear scientists 1 ITS Advisory Council 5 Seaborg the scientist 7 Naming seaborgium 11 Branching out 13 IGCAR honors Seaborg’s contributions to actinide science 16 Seaborg Institute for Transactinium Science/Los Alamos National Laboratory Actinide Research Quarterly The Seaborg Institute for Transactinium Science Established to educate the next generation of nuclear scientists In the late 1970s and early 1980s, research opportunities in heavy element This article was contributed by science and engineering were seldom found in university settings, and the supply Darleane C. Hoffman, professor emerita, of professionals in those fields was diminishing to the detriment of national Graduate School, Department of goals. In response to these concerns, national studies were conducted and panels Chemistry, UC Berkeley, faculty senior and committees were formed to assess the status of training and education in the scientist, Lawrence Berkeley National nuclear sciences. The studies in particular addressed the future need for scientists Laboratory, and charter director, Seaborg trained in the areas of nuclear waste management and disposal, environmental Institute, Lawrence Livermore National remediation, nuclear fuel processing, and nuclear safety analysis. Laboratory; and Christopher Gatrousis, Unfortunately, with the exception of the American Chemical Society’s former associate director of Chemistry Division of Nuclear Chemistry and Technology summer schools for undergradu- and Materials Science, Lawrence ates in nuclear and radiochemistry established in 1984, the studies did not lead Livermore National Laboratory. -

On the Ontology of Superheavy Elements
On the ontology of superheavy elements Kragh, Helge Stjernholm Published in: Substantia DOI: 10.13128/substantia-25 Publication date: 2017 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Kragh, H. S. (2017). On the ontology of superheavy elements. Substantia, 1(2), 7-17. https://doi.org/10.13128/substantia-25 Download date: 24. sep.. 2021 Firenze University Press www.fupress.com/substantia Research Article On the Ontology of Superheavy Elements Citation: H. Kragh (2017) On the Ontology of Superheavy Elements. Helge Kragh Substantia 1(2): 7-17. doi: 10.13128/ substantia-25 Niels Bohr Institute, University of Copenhagen, Blegdamsvej 17, Copenhagen, Denmark E-mail: [email protected] Copyright: © 2017 H. Kragh. This is an open access, peer-reviewed article published by Firenze University Press Abstract. The study of so-called superheavy elements with atomic numbers Z > 102 (http://www.fupress.com/substantia) has for several decades been a major research field in nuclear physics and chemistry. and distribuited under the terms of the Presently all elements up to and including Z = 118 have been discovered and assigned Creative Commons Attribution License, official names by IUPAC. To speak of “discovery” is however unfortunate since the ele- which permits unrestricted use, distri- ments are in reality produced, manufactured or created in the laboratory. They are not bution, and reproduction in any medi- found in nature. Moreover, it is not obvious that they exist in the normal sense of the um, provided the original author and source are credited. -

NEW ELEMENT, LAWRENCIUM, ATOMIC NUMBER 103 Albert Ghiorso, Torbjgrn Sikkeland, Almon E
VOLUME 6) NUMBER 9 PHYSICAL REVIEW LETTERS Mav l, 1961 NEW ELEMENT, LAWRENCIUM, ATOMIC NUMBER 103 Albert Ghiorso, Torbjgrn Sikkeland, Almon E. Larsh, and Robert M. Latimer Lawrence Radiation Laboratory, University of California, Berkeley, California (Received April 13, 1961) Bombardments of californium with boron ions because heavy-ion bombardment of these elements have produced alpha-particle activity which can produces in high yield an alpha activity with an only be ascribed to decay of a new element with 8.8-Mev alpha-particle energy and a 25-second atomic number 103. This activity at best amounts half-life which can obscure the lower energy to only a few alpha counts per hour (@=1 micro- alpha activity of element 103. The heavy-ion barn), so it has not yet been possible to detect beam of either B' or B"was collimated so as the mendelevium decay product to prove further to pass through the tiny target and typically was the atomic number of the new activity. For the limited to 0.5 microampere dc to avoid melting present, attribution of this activity to element the target foil. The transmuted atoms recoiled number 103 must rest entirely on nuclear rather from the target into an atmosphere of helium. than chemical evidence. This gas flowed slowly through a nearby 0.050- The method used to produce and identify radi- inch orifice and carried the electrically charged ations from element 103 decay is shown schemat- transmutation products to a thin copper conveyor ically in Fig. 1 and is based on the one used for tape. -
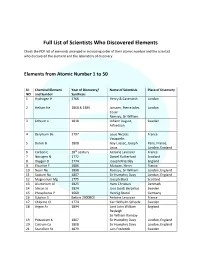
Full List of Scientists Who Discovered Elements
Full List of Scientists Who Discovered Elements Check the PDF list of elements arranged in increasing order of their atomic number and the scientist who discovered the element and the laboratory of discovery. Elements from Atomic Number 1 to 50 SI. Chemical Element Year of Discovery/ Name of Scientists Place of Discovery NO and Symbol Synthesis 1 Hydrogen H 1766 Henry & Cavendish London 2 Helium He 1868 & 1895 Janssen, Pierre Jules London Cesar Ramsay, Sir William 3 Lithium Li 1818 Johann August, Sweden Arfvedson 4 Beryllium Be 1797 Louis Nicolas France Vauquelin 5 Boron B 1808 Gay-Lussac, Joseph Paris, France, Louis London, England 6 Carbon C 18th century Antoine Lavoisier France 7 Nitrogen N 1772 Daniel Rutherford Scotland 8 Oxygen O 1774 Joseph Priestley England 9 Fluorine F 1886 Moissan, Henri France 10 Neon Ne 1898 Ramsay, Sir William London, England 11 Sodium Na 1807 Sir Humphry Davy London, England 12 Magnesium Mg 1775 Joseph Black Scotland 13 Aluminium Al 1825 Hans Christian Denmark 14 Silicon Si 1824 Jons Jacob Berzelius Sweden 15 Phosphorus P 1669 Hennig Brand Germany 16 Sulphur S Before 2000BCE Antoine Lavoisier France 17 Chlorine Cl 1774 Carl Wilhelm Scheele Sweden 18 Argon Ar 1894 Lord John William England Rayleigh Sir William Ramsay 19 Potassium K 1807 Sir Humphry Davy London, England 20 Calcium Ca 1808 Sir Humphry Davy London, England 21 Scandium Sc 1879 Lars Frederick Sweden 22 Titanium Ti 1791, 1795 William Gregor England, Germany Martin Heinrich 23 Vanadium V 1801 Andrés Manuel Mexico 24 Chromium Cr 1797 Louis-Nicholas -

The Life and Contributions to the Periodic Table of Glenn T. Seaborg
Pure Appl. Chem. 2019; 91(12): 1929–1939 Conference paper David Seaborg* The life and contributions to the periodic table of Glenn T. Seaborg, the first person to have an element named after him while he was still alive https://doi.org/10.1515/pac-2019-0816 Abstract: Glenn Seaborg was born on Michigan’s Upper Peninsula in 1912. At age 10, he moved to southern California, where his high school chemistry and physics teacher, Dwight Logan Read, sparked his interest in these fields. Seaborg was the first person to have an element named after him while he was still living. The only other person to have an element named after him while he was still alive was Yuri Oganessian. Keywords: actinide series; Mendeleev 150; periodic system; periodic table; Seaborg. Early life Glenn Theodore Seaborg was born in Ishpeming, on Michigan’s Upper Peninsula, in the United States, on April 19, 1912. His parents were Swedish Americans, and he spoke Swedish before he spoke English. He was proud of his Swedish heritage for his entire life. Ishpeming was rural. It has a cold climate similar to that of Sweden, which is why Seaborg’ paternal grandfather and mother each moved there from Sweden. The snow was so high in winter that he skied out of the second story window of his modest house. In his 10 years in Ishpeming, he never talked on the phone or heard the word “radio”. He never forgot his roots in Ishpeming, and visited it throughout his life. The Seaborg’s friends Bob and Ruth Engstroms had moved to Southern California and wrote a letter urging Glenn’s parents to join them. -
Lawrence Berkeley Laboratory UNIVERSITY of CALIFORNIA
LBL-9074 :!"4 Preprint Lawrence Berkeley Laboratory UNIVERSITY OF CALIFORNIA 1l Published in Chemical and Engineering News, Vol. 57, pp. 46-52. April 16, 1979 THE PERIODIC TABLE TORTUOUS PATH TO MAN-MADE ELEMENTS Glenn T. Seaborg rhE Y L.OATQ!Y AUG 2E 1979 April 1979 L1BR/RY 1.ND DOCUMENTS SEc.iN TWO-WEEK LOAN COPY This is a Library Circulating Copy which may be borrowed for two weeks. For a personal retention copy, call Tech. Info. DIvision, Ext. 6782 (1 Ill IT T 1 Vjs%0 Prepared for the U. S. Department of Energy under Contract W-7405-ENG-48 DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. -
If It Made Chemistry News, You'll Find It
If It Made Chemistry News, You’ll Find It in… QUESTION: Why was the naming of elements 104, 105, and 106 so controversial? Search Citation DOI Advanced Search elements 104 OR 105 OR 106 Anywhere Search FinDinG THE ANSweR IS QUICK AND EASY Using the Quick Search box at the C&EN Archives homepage (pubs.acs.org/cen-archives), you can perform a Boolean search for elements 104 OR 105 OR 106. This produces a lengthy list of hits; however, the first hit on the list begins to answer your question and quickly guides you to the other articles that you need. Researching element 104, you’ll discover that… “…the element, which has the atomic number 104, was first reported by Georgii Flerov and colleagues at the Joint Institute for Nuclear Research in Dubna, Russia, in 1964. The Russian scientists named the element ‘kurchatovium’ in honor of nuclear physicist Igor Kurchatov…”1 IT'S ELEMENTAL! The article goes on to note… RUTHERFORDIUM AT A GLANCE “Glenn T. Seaborg, Albert Ghiorso, and coworkers at RUTHERFORDIUM Name: Named after New Zealand MICHAEL FREEMANTLE, C&EN LONDON physicist Ernest Rutherford. Atomic mass: (261). Lawrence Berkeley National Laboratory considered the UTHERFORDIUM IS AN ELEMENT is the Latin word code for 104 (un = 1, nil = History: Production first reported by a more famed for its names than 0, and quad = 4). Its symbol was "Unq." team at the Joint Institute for Nuclear its properties or uses. It was al- Seaborg thought these IUPAC names Research in Dubna, Russia, in 1964 Al- so my baptism of fire into the in- were "unnecessarily cumbersome" and bert Ghiorso and his team at the Univer- Dubna discovery in 1964 to be invalid, saying it was based ternational politics and sensi- served "no useful purpose" (C&EN, May 13, sity of California, Berkeley, produced a tivities of naming new elements. -

1 H 2 He 4 Be 5 B 6 C 7 N 11 Na 3 Li 8 O 9 F 10 Ne 12 Mg 13 Al 14 Si 15
1 2 H He Hydrogen Helium Discovered 1766 Discovered 1895 ` From the Greek From the Greek for water-forming for sun, 1st found in the sun’s corona 3 4 5 6 7 8 9 10 Li Be B C N O F Ne Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon Discovered 1817 Discovered 1797 Discovered 1808 Prehistoric Discovered 1772 Discovered 1774 Discovered 1886 Discovered 1898 ` From the Greek From the Greek From the Arabic From the Latin From the Greek From the Greek From the Latin From the Greek “lithos” (stone) for beryl, a “burqa” (borax) “carbo” (charcoal) for nitre-forming for acid-forming “fluere” (to flow) “neos” (new) gemstone 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon Discovered 1807 Discovered 1755 Discovered 1825 Discovered 1824 Discovered 1669 Prehistoric Discovered 1774 Discovered 1894 ` From the English For Magnesia, a From the Latin From the Latin From the Greek Sanskrit “sulvere” From the Greek From the Greek “soda” district in Thessaly, “alum” “silex” (flint) for light-bringing or Latin “chloros” “argos” (idle) Greece (bitter salt) “sulfurium” (greenish yellow) 19 20 31 32 33 34 35 36 K CA Ga Ge As Se Br Kr Potassium Calcium Gallium Germanium Arsenic Selenium Bromine Krypton Discovered 1807 Discovered 1808 Discovered 1875 Discovered 1886 Discovered ~1250 Discovered 1817 Discovered 1826 Discovered 1898 ` From the English From the Latin From the Latin From the Latin From the Greek From the Greek From the Greek From the Greek “potash” “calex” (lime) name for France name for Germany “arsenikon” (a name for the “bromos” “kryptos” (hidden) (“Gallia”) (“Germania”) yellow pigment) moon (“Selene”) (stench) 1 Henry Cavendish 2 Helium was de- discovered hydro- tected in the sun by gen. -

National Medal of Science
Albert Ghiorso 1915-2010 BERKELEY, CA. – Albert Ghiorso, lifelong nuclear researcher at the Lawrence Berkeley Laboratory, the co-discoverer of twelve chemical elements, more than anyone else in history, and a prolific inventor of nuclear technology, died December 26, 2010, at the age of 95. Ghiorso’s discoveries include the following elements: Atomic Name Year number 95 Americium 1945 96 Curium 1944 97 Berkelium 1949 98 Californium 1950 99 Einsteinium 1952 100 Fermium 1953 101 Mendelevium 1955 102 Nobelium 1958 103 Lawrencium 1961 104 Rutherfordium 1969 105 Dubnium 1970 106 Seaborgium 1974 Over a period of more than 50 years, Ghiorso led the development of major new techniques in nuclear science, enabling the discovery about 30 new isotopes of 12 new Albert Ghiorso 1915-2010 Short version (1200 words) Updated 11 Jan 2011 Page 1 of 5 elements. This work was central to proving the correctness of Seaborg’s theoretical revision of the periodic chart and its extension to higher atomic numbers, which in turn directly and significantly impacted all of nuclear chemistry, physics, weapons, research, medical treatment of cancer, and consumer products. Albert Ghiorso was born in 1915 in Vallejo, California, of Italian and Spanish ancestry. As a child, he developed a fascination with airplanes, machines, and radio. As a teenager, he built short-wave radios that out-performed military equipment. After obtaining his B.S. degree in engineering from the University of California in 1937, he built specialized radio gear, and through that work came into contact with the Radiation Laboratory on the Berkeley campus. This led to his employment designing and building the world’s first commercial Geiger counters to detect nuclear radiation.