Press Release
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View a Copy of This Licence, Visit Tivecommons.Org/Licenses/By/4.0
Katakami et al. Cardiovasc Diabetol (2020) 19:110 https://doi.org/10.1186/s12933-020-01079-4 Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Tofoglifozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study Naoto Katakami1,2* , Tomoya Mita3, Hidenori Yoshii4, Toshihiko Shiraiwa5, Tetsuyuki Yasuda6, Yosuke Okada7, Keiichi Torimoto7, Yutaka Umayahara8, Hideaki Kaneto9, Takeshi Osonoi10, Tsunehiko Yamamoto11, Nobuichi Kuribayashi12, Kazuhisa Maeda13, Hiroki Yokoyama14, Keisuke Kosugi15, Kentaro Ohtoshi16, Isao Hayashi17, Satoru Sumitani18, Mamiko Tsugawa19, Kayoko Ryomoto20, Hideki Taki21, Tadashi Nakamura22, Satoshi Kawashima23, Yasunori Sato24, Hirotaka Watada3 and Iichiro Shimomura1 on behalf of the UTOPIA study investigators Abstract Background: This study aimed to investigate the preventive efects of tofoglifozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression in type 2 diabetes (T2DM) patients without apparent cardiovascular disease (CVD) by monitoring carotid intima-media thickness (IMT). Methods: This prospective, randomized, open-label, blinded-endpoint, multicenter, parallel-group, comparative study included 340 subjects with T2DM and no history of apparent CVD recruited at 24 clinical units. Subjects were randomly allocated to either the tofoglifozin treatment group (n 169) or conventional treatment group using drugs other than SGLT2 inhibitors (n 171). Primary outcomes were changes= in mean and maximum common carotid IMT measured by echography during= a 104-week treatment period. Results: In a mixed-efects model for repeated measures, the mean IMT of the common carotid artery (mean- IMT-CCA), along with the right and left maximum IMT of the CCA (max-IMT-CCA), signifcantly declined in both the tofoglifozin ( 0.132 mm, SE 0.007; 0.163 mm, SE 0.013; 0.170 mm, SE 0.020, respectively) and the control group ( 0.140 mm,− SE 0.006; 0.190 mm,− SE 0.012; 0.190 mm,− SE 0.020, respectively). -

Daiichi Sankyo Company, Limited
[Translation] CONVOCATION NOTICE OF THE 14TH ORDINARY GENERAL MEETING OF SHAREHOLDERS For the Fiscal Year Ended March 31, 2019 Daiichi Sankyo Company, Limited *Note: This translation does not include pictures, charts etc. originally issued in the Japanese version. - 1 - [Translation] To Our Shareholders At the Daiichi Sankyo Group (“the Group”), we are proceeding with the initiatives of 4th mid- term business plan with the aim of becoming a “Global Pharma Innovator with Competitive Advantage in Oncology” as set forth in our 2025 Vision. In fiscal 2018, we made significant progress in developing new drugs in oncology area, including DS-8201, an antibody drug conjugates utilizing our proprietary technologies. In addition, we entered into a strategic collaboration agreement with AstraZeneca, which has strengths in the oncology business, for the global development and commercialization of DS-8201 in order to maximize its value. Furthermore, sales of mainstay products such as edoxaban, an anticoagulant which supports current earnings of the Group, were firm in Japan and overseas. The achievement of initial target of mid-term business plan for fiscal 2020 is expected to be delayed by two years due to failure of achievement of the plan for pain franchise business and additional investments in research and development. However, we are gaining confidence of achieving our 2025 Vision and accelerating growth in the future due to the significant improvement in the value of oncology area pipelines. We will continue to make every effort to achieve the goals of the Medium-Term Management Plan and 2025 Vision. I greatly appreciate your continued support in the future. -

Daiichi Sankyo Group Value Report 2019
External Evaluations (as of June 30,2019) ™ Daiichi Sankyo Group Value Report 2019 Value Daiichi Sankyo Group MSCI Japan Empowering Women Select Index THE INCLUSION OF DAIICHI SANKYO CO.,LTD. IN ANY MSCI INDEX, AND THE USE OF MSCI LOGOS, TRADEMARKS, SERVICE MARKS OR INDEX NAMES HEREIN, DO NOT CONSTITUTE A SPONSORSHIP, ENDORSEMENT OR PROMOTION OF DAIICHI SANKYO CO.,LTD. BY MSCI OR ANY OF ITS AFFILIATES. THE MSCI INDEXES ARE THE EXCLUSIVE PROPERTY OF MSCI. MSCI AND THE MSCI INDEX NAMES AND LOGOS ARE TRADE- MARKS OR SERVICE MARKS OF MSCI OR ITS AFFILIATES. “Eruboshi” Certification Mark “Kurumin” Certification Mark Logo given to Certified Health and Productivity Management Organization (White500) This report uses FSC® certified paper, which indicates that the paper used to print this Paper report was produced from properly managed forests. 3-5-1, Nihonbashi-honcho, Chuo-ku, Tokyo 103-8426, Japan This report was printed using 100% Inks biodegradable printing inks from vegetable Corporate Communications Department oil. Daiichi Sankyo Group Tel: +81-3-6225-1126 CSR Department The waterless printing method used for this Value Report 2019 Tel: +81-3-6225-1067 Printing report minimized the use and release of harmful liquid wastes. https://www.daiichisankyo.com/ Printed in Japan 005_7045687911909.indd 1 2019/09/27 18:22:19 Introduction Our Mission The Core Values and Commitments serve as the criteria for business activities and In addition, we have established the DAIICHI SANKYO Group Corporate Conduct Charter . decision-making used by executive officers and employees in working to fulfill Our Mission . This charter calls on us to fulfill our social responsibilities by acting with the highest ethical Our Corporate Slogan succinctly explains the spirit of Our Mission, Core Values and standards and a good social conscience appropriate for a company engaged in business Commitments. -

Convocation Notice of the 16Th Ordinary General Meeting of Shareholders
[Translation] CONVOCATION NOTICE OF THE 16TH ORDINARY GENERAL MEETING OF SHAREHOLDERS For the Fiscal Year Ended March 31, 2021 Daiichi Sankyo Company, Limited *Note: This translation does not include pictures, charts etc. originally issued in the Japanese version. [Translation] To Our Shareholders We sincerely appreciate the continuous kindness of our shareholders. In addition, we would like to express our deepest sympathies to those who passed away due to COVID-19, and thank the medical personnel who are close to those who are fighting illness and are making efforts in treatment. We will continue to devote ourselves to the research and development of vaccines and therapeutic agents. Our “Purpose” is to “contribute to the enrichment of quality of life around the world.” As a pharmaceutical company with strengths in science and technology, we continuously create innovative pharmaceuticals and provide pharmaceuticals that meet diverse medical needs to provide sustainable value to society. We were able to launch the anti-cancer drug “Enhertu”, which is an antibody-drug conjugate (ADC) that utilizes our unique technology, in Japan and Europe in fiscal 2020, following the launch in the U.S. in fiscal 2019. Subsequent ADCs such as Dato-DXd and HER3-DXd are also steadily developing. Now, we have newly established our 2030 Vision of being an “Innovative Global Healthcare Company Contributing to the Sustainable Development of Society,” and have established 5-Year Business Plan (fiscal 2021 to fiscal 2025) as a plan to realize our 2025 Vision, “Global Pharma Innovator with Competitive Advantage in Oncology.” By working together as a Daiichi Sankyo Group on 5-Year Business Plan toward the 2030 Vision, we aim to solve the social issues expected of our company and increase shareholder value. -

Daiichi Sankyo Company, Limited
[Translation] CONVOCATION NOTICE OF THE 6TH ORDINARY GENERAL MEETING OF SHAREHOLDERS For the Fiscal Period Ended March 31, 2011 Daiichi Sankyo Company, Limited - 1 - [Translation] (Securities Identification Code 4568) May 31, 2011 To Shareholders, Daiichi Sankyo Company, Limited Joji Nakayama, Representative Director and President & CEO 5-1, Nihonbashi Honcho 3-chome, Chuo-ku, Tokyo, Japan CONVOCATION NOTICE OF THE 6TH ORDINARY GENERAL MEETING OF SHAREHOLDERS We wish to extend our deepest sympathy to all those who have suffered hardship from the Great East Japan Earthquake that occurred in March 2011. Daiichi Sankyo Company, Limited (“the Company”) respectfully requests your attendance at the 6th Ordinary General Meeting of Shareholders (“the Meeting”), which will be held as detailed below. If you will not be able to attend the Meeting, you may exercise your voting rights through either of the methods described below, in which case we ask that you please exercise your voting rights by 17:30 (within our business hours), Friday, June 24, 2011 (Japan Time), after examining the attached reference documents. [Exercise of Voting Rights by Mail] Please indicate your approval or disapproval for the proposals on the enclosed voting form and return the form to the Company. Please note that the form must be received by the Company no later than the above-mentioned deadline. [Exercise of Voting Rights on the Internet etc.] After examining “Information on Exercise of Voting Rights, etc.” on pages 57 and 58, please vote on the Internet at the dedicated voting website (http://www.evote.jp/) no later than the above-mentioned deadline. The Company is participating in the platform for electronic exercise of voting rights for institutional investors operated by ICJ Inc. -

Ranking of Stocks by Market Capitalization(As of End of Mar.2020)
Ranking of Stocks by Market Capitalization(As of End of Mar.2020) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 212,127 2 9437 NTT DOCOMO,INC. 112,630 3 6861 KEYENCE CORPORATION 84,709 4 6758 SONY CORPORATION 81,786 5 9984 SoftBank Group Corp. 79,162 6 9433 KDDI CORPORATION 75,136 7 4519 CHUGAI PHARMACEUTICAL CO.,LTD. 69,960 8 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 68,006 9 9434 SoftBank Corp. 65,799 10 7974 Nintendo Co.,Ltd. 54,787 11 8306 Mitsubishi UFJ Financial Group,Inc. 54,735 12 4568 DAIICHI SANKYO COMPANY,LIMITED 52,707 13 4502 Takeda Pharmaceutical Company Limited 52,146 14 4661 ORIENTAL LAND CO.,LTD. 50,261 15 6098 Recruit Holdings Co.,Ltd. 47,419 16 9983 FAST RETAILING CO.,LTD. 46,873 17 7182 JAPAN POST BANK Co.,Ltd. 44,865 18 4063 Shin-Etsu Chemical Co.,Ltd. 44,707 19 7267 HONDA MOTOR CO.,LTD. 44,017 20 4452 Kao Corporation 42,560 21 6367 DAIKIN INDUSTRIES,LTD. 38,603 22 6981 Murata Manufacturing Co.,Ltd. 36,980 23 8058 Mitsubishi Corporation 36,436 24 8316 Sumitomo Mitsui Financial Group,Inc. 36,018 25 9022 Central Japan Railway Company 35,679 26 8001 ITOCHU Corporation 35,541 27 8766 Tokio Marine Holdings,Inc. 35,145 28 7741 HOYA CORPORATION 34,808 29 6594 NIDEC CORPORATION 33,433 30 8035 Tokyo Electron Limited 32,000 31 3382 Seven & I Holdings Co.,Ltd. 31,699 32 7751 CANON INC. 31,463 33 8411 Mizuho Financial Group,Inc. -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -
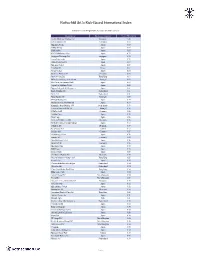
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Association Between Variability in Body Mass Index and Development of Type 2 Diabetes: Panasonic Cohort Study
Epidemiology/Health services research Open access Original research BMJ Open Diab Res Care: first published as 10.1136/bmjdrc-2021-002123 on 22 April 2021. Downloaded from Association between variability in body mass index and development of type 2 diabetes: Panasonic cohort study Hiroshi Okada ,1 Masahide Hamaguchi ,2 Momoko Habu,1 Kazushiro Kurogi,3 Hiroaki Murata,4 Masato Ito,3 Michiaki Fukui2 To cite: Okada H, ABSTRACT Hamaguchi M, Habu M, Introduction Contrasting results have been reported for Significance of this study et al. Association between the association between the variability in body weight variability in body mass index and development of diabetes. In the present study, we What is already known about this subject? and development of type 2 evaluated the association between the variability in body ► Obesity or body weight gain accelerate the develop- diabetes: Panasonic cohort ment of diabetes. study. BMJ Open Diab Res Care mass index (BMI) and development of type 2 diabetes in 19 412 Japanese participants without obesity and without ► The association between the variability in body 2021;9:e002123. doi:10.1136/ weight and development of diabetes is controversial. bmjdrc-2021-002123 body weight gain or loss during the study period. Research design and methods We recorded body weight What are the new findings? of the participants consecutively each year in Panasonic ► The risk for developing diabetes increased by 11.1% Received 9 January 2021 Corporation, Osaka, Japan from 2008 to 2014 to evaluate Revised 26 February 2021 per 1% increase in coefficient of variation of body the variability of BMI. The participants with obesity (BMI mass index (BMI). -

Daiichi Sankyo Group Value Report 2016
Daiichi Sankyo Group Value Report 2016 Daiichi Sankyo Group Value Daiichi Sankyo Group Value Report 2016 Our Mission Core Values and Commitments(Criteria of the Value Judgment to Fulfill Our Mission) Core Values Notes To contribute to the enrichment of quality of life around the world Innovation : the introduction of new ideas, methods, or invention through the creation of innovative pharmaceuticals, and through the Integrity : the quality of being honest and of always having high moral principles provision of pharmaceuticals addressing diverse medical needs. Accountability : being responsible for the effects of your actions, and being willing to explain or be criticized for them Commitments We have established Core Values and Commitments as the criteria for our business activities 1. To create innovative medicines changing SOC * and decision making. Our global brand is a pledge to our stakeholders of what the * SOC (Standard of Care): Universally applied best treatment practice in today’s medical science Company is capable of delivering, now and in the future. Our corporate slogan succinctly 2. To take a global perspective, and respect regional values states how we make efforts for what and for whom. 3. To foster intellectual curiosity and strategic insight In addition, we have established the DAIICHI SANKYO Group Corporate Conduct Charter * to act with the highest ethical standards and a good social conscience appropriate 4. To provide the highest quality medical information for a company engaged in a business that affects human lives. 5. To provide a stable supply of top-quality pharmaceutical products * The full text of the DAIICHI SANKYO Group Corporate Conduct Charter can be found on page 28. -

Notice of the Ordinary General Meeting of Shareholders to Be Held on June 26, 2020
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 26, 2020 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 26, 2020 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To amend a part of the Articles of Incorporation. 2. To elect 12 Directors. 3. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020). Note: The Consolidated Financial Statements pursuant to the Companies Act of Japan (Translation) are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting103/ 1 PROPOSALS TO BE ACTED UPON: 1. -

Summary Conflict of Interest Statements BCC 2021
Summary conflict of interest statements BCC 2021 Last name First name Type of affiliation/ financial interest Name of commercial company Aapro Matti Receipt of grants/research supports: Amgen, Eisai, Genomic Health, Helsinn, Hospira, Novartis, Merck, Mundipharma, Pfizer, Rache, Sandoz, Tesaro, Teva, Vifor Receipt of honoraria or consultation fees: Accord Pharmaceuticals, Amgen, Astellas, Bayer HealthCare Pharmaceuticals (Schering), Biocon, Boehringer Ingelheim, BMS, Celgene, Cephalon, Chugai Pharmaceutical Co. Ltd., Clinigen Group, Dr.Reddy's Laboratories, Eisai Co. Ltd., Eli Lilly, Genomic Health (Exact Sciences), GlaxoSmithKline (GSK), Glenmark Pharmaceuticals Limited, Gl Therapeutics, lnc., Helsinn Healthcare SA, Hospira (Pfizer), lpsen, Janssen Biotech, Johnson & Johnson, Kyowa Kirin Group, Merck, Merck Serono (Merck KGaA), Mundipharma International Limited, Novartis, Pfizer, Pierre Fabre, Rache, Sandoz, Sanofi, Taiho Pharmaceutical, Tesaro (GSK), Teva Pharmaceutical lndustries Ltd., Vifor Pharma Other support: European Cancer Organisation, SPCC, Cancer Center Genolier Aebi Stephan Receipt of honoraria or consultation fees: Novartis, Roche, Pfizer Other support: Support for CME lectures of the Lucerne Cancer Center: Amgen, Astellas, Bayer, Bristol-Myers Squibb, Debiopharm International SA, Eisai, Ipsen Pharma, Janssen, Merck, MSD, Pfizer, Roche, Sanofi Genzyme, Servier, Takeda André Fabrice Receipt of grants/research supports: Comepensated to the hospital: Roche, AstraZeneca, Daiichi Sankyo, Pfizer, Novartis, Lilly Barrios Carlos