Daiichi Sankyo Company, Limited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Daiichi Sankyo Group Value Report 2019
External Evaluations (as of June 30,2019) ™ Daiichi Sankyo Group Value Report 2019 Value Daiichi Sankyo Group MSCI Japan Empowering Women Select Index THE INCLUSION OF DAIICHI SANKYO CO.,LTD. IN ANY MSCI INDEX, AND THE USE OF MSCI LOGOS, TRADEMARKS, SERVICE MARKS OR INDEX NAMES HEREIN, DO NOT CONSTITUTE A SPONSORSHIP, ENDORSEMENT OR PROMOTION OF DAIICHI SANKYO CO.,LTD. BY MSCI OR ANY OF ITS AFFILIATES. THE MSCI INDEXES ARE THE EXCLUSIVE PROPERTY OF MSCI. MSCI AND THE MSCI INDEX NAMES AND LOGOS ARE TRADE- MARKS OR SERVICE MARKS OF MSCI OR ITS AFFILIATES. “Eruboshi” Certification Mark “Kurumin” Certification Mark Logo given to Certified Health and Productivity Management Organization (White500) This report uses FSC® certified paper, which indicates that the paper used to print this Paper report was produced from properly managed forests. 3-5-1, Nihonbashi-honcho, Chuo-ku, Tokyo 103-8426, Japan This report was printed using 100% Inks biodegradable printing inks from vegetable Corporate Communications Department oil. Daiichi Sankyo Group Tel: +81-3-6225-1126 CSR Department The waterless printing method used for this Value Report 2019 Tel: +81-3-6225-1067 Printing report minimized the use and release of harmful liquid wastes. https://www.daiichisankyo.com/ Printed in Japan 005_7045687911909.indd 1 2019/09/27 18:22:19 Introduction Our Mission The Core Values and Commitments serve as the criteria for business activities and In addition, we have established the DAIICHI SANKYO Group Corporate Conduct Charter . decision-making used by executive officers and employees in working to fulfill Our Mission . This charter calls on us to fulfill our social responsibilities by acting with the highest ethical Our Corporate Slogan succinctly explains the spirit of Our Mission, Core Values and standards and a good social conscience appropriate for a company engaged in business Commitments. -

Convocation Notice of the 16Th Ordinary General Meeting of Shareholders
[Translation] CONVOCATION NOTICE OF THE 16TH ORDINARY GENERAL MEETING OF SHAREHOLDERS For the Fiscal Year Ended March 31, 2021 Daiichi Sankyo Company, Limited *Note: This translation does not include pictures, charts etc. originally issued in the Japanese version. [Translation] To Our Shareholders We sincerely appreciate the continuous kindness of our shareholders. In addition, we would like to express our deepest sympathies to those who passed away due to COVID-19, and thank the medical personnel who are close to those who are fighting illness and are making efforts in treatment. We will continue to devote ourselves to the research and development of vaccines and therapeutic agents. Our “Purpose” is to “contribute to the enrichment of quality of life around the world.” As a pharmaceutical company with strengths in science and technology, we continuously create innovative pharmaceuticals and provide pharmaceuticals that meet diverse medical needs to provide sustainable value to society. We were able to launch the anti-cancer drug “Enhertu”, which is an antibody-drug conjugate (ADC) that utilizes our unique technology, in Japan and Europe in fiscal 2020, following the launch in the U.S. in fiscal 2019. Subsequent ADCs such as Dato-DXd and HER3-DXd are also steadily developing. Now, we have newly established our 2030 Vision of being an “Innovative Global Healthcare Company Contributing to the Sustainable Development of Society,” and have established 5-Year Business Plan (fiscal 2021 to fiscal 2025) as a plan to realize our 2025 Vision, “Global Pharma Innovator with Competitive Advantage in Oncology.” By working together as a Daiichi Sankyo Group on 5-Year Business Plan toward the 2030 Vision, we aim to solve the social issues expected of our company and increase shareholder value. -

Daiichi Sankyo Company, Limited
[Translation] CONVOCATION NOTICE OF THE 6TH ORDINARY GENERAL MEETING OF SHAREHOLDERS For the Fiscal Period Ended March 31, 2011 Daiichi Sankyo Company, Limited - 1 - [Translation] (Securities Identification Code 4568) May 31, 2011 To Shareholders, Daiichi Sankyo Company, Limited Joji Nakayama, Representative Director and President & CEO 5-1, Nihonbashi Honcho 3-chome, Chuo-ku, Tokyo, Japan CONVOCATION NOTICE OF THE 6TH ORDINARY GENERAL MEETING OF SHAREHOLDERS We wish to extend our deepest sympathy to all those who have suffered hardship from the Great East Japan Earthquake that occurred in March 2011. Daiichi Sankyo Company, Limited (“the Company”) respectfully requests your attendance at the 6th Ordinary General Meeting of Shareholders (“the Meeting”), which will be held as detailed below. If you will not be able to attend the Meeting, you may exercise your voting rights through either of the methods described below, in which case we ask that you please exercise your voting rights by 17:30 (within our business hours), Friday, June 24, 2011 (Japan Time), after examining the attached reference documents. [Exercise of Voting Rights by Mail] Please indicate your approval or disapproval for the proposals on the enclosed voting form and return the form to the Company. Please note that the form must be received by the Company no later than the above-mentioned deadline. [Exercise of Voting Rights on the Internet etc.] After examining “Information on Exercise of Voting Rights, etc.” on pages 57 and 58, please vote on the Internet at the dedicated voting website (http://www.evote.jp/) no later than the above-mentioned deadline. The Company is participating in the platform for electronic exercise of voting rights for institutional investors operated by ICJ Inc. -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -
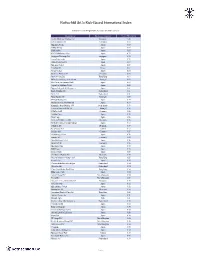
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Daiichi Sankyo Group Value Report 2016
Daiichi Sankyo Group Value Report 2016 Daiichi Sankyo Group Value Daiichi Sankyo Group Value Report 2016 Our Mission Core Values and Commitments(Criteria of the Value Judgment to Fulfill Our Mission) Core Values Notes To contribute to the enrichment of quality of life around the world Innovation : the introduction of new ideas, methods, or invention through the creation of innovative pharmaceuticals, and through the Integrity : the quality of being honest and of always having high moral principles provision of pharmaceuticals addressing diverse medical needs. Accountability : being responsible for the effects of your actions, and being willing to explain or be criticized for them Commitments We have established Core Values and Commitments as the criteria for our business activities 1. To create innovative medicines changing SOC * and decision making. Our global brand is a pledge to our stakeholders of what the * SOC (Standard of Care): Universally applied best treatment practice in today’s medical science Company is capable of delivering, now and in the future. Our corporate slogan succinctly 2. To take a global perspective, and respect regional values states how we make efforts for what and for whom. 3. To foster intellectual curiosity and strategic insight In addition, we have established the DAIICHI SANKYO Group Corporate Conduct Charter * to act with the highest ethical standards and a good social conscience appropriate 4. To provide the highest quality medical information for a company engaged in a business that affects human lives. 5. To provide a stable supply of top-quality pharmaceutical products * The full text of the DAIICHI SANKYO Group Corporate Conduct Charter can be found on page 28. -

Summary Conflict of Interest Statements BCC 2021
Summary conflict of interest statements BCC 2021 Last name First name Type of affiliation/ financial interest Name of commercial company Aapro Matti Receipt of grants/research supports: Amgen, Eisai, Genomic Health, Helsinn, Hospira, Novartis, Merck, Mundipharma, Pfizer, Rache, Sandoz, Tesaro, Teva, Vifor Receipt of honoraria or consultation fees: Accord Pharmaceuticals, Amgen, Astellas, Bayer HealthCare Pharmaceuticals (Schering), Biocon, Boehringer Ingelheim, BMS, Celgene, Cephalon, Chugai Pharmaceutical Co. Ltd., Clinigen Group, Dr.Reddy's Laboratories, Eisai Co. Ltd., Eli Lilly, Genomic Health (Exact Sciences), GlaxoSmithKline (GSK), Glenmark Pharmaceuticals Limited, Gl Therapeutics, lnc., Helsinn Healthcare SA, Hospira (Pfizer), lpsen, Janssen Biotech, Johnson & Johnson, Kyowa Kirin Group, Merck, Merck Serono (Merck KGaA), Mundipharma International Limited, Novartis, Pfizer, Pierre Fabre, Rache, Sandoz, Sanofi, Taiho Pharmaceutical, Tesaro (GSK), Teva Pharmaceutical lndustries Ltd., Vifor Pharma Other support: European Cancer Organisation, SPCC, Cancer Center Genolier Aebi Stephan Receipt of honoraria or consultation fees: Novartis, Roche, Pfizer Other support: Support for CME lectures of the Lucerne Cancer Center: Amgen, Astellas, Bayer, Bristol-Myers Squibb, Debiopharm International SA, Eisai, Ipsen Pharma, Janssen, Merck, MSD, Pfizer, Roche, Sanofi Genzyme, Servier, Takeda André Fabrice Receipt of grants/research supports: Comepensated to the hospital: Roche, AstraZeneca, Daiichi Sankyo, Pfizer, Novartis, Lilly Barrios Carlos -

Senior Executives
Senior Executives Mizuho Financial Group Masayuki Hoshi Tsuyoshi Hatsuzawa Managing Executive Officer Managing Executive Officer Directors and Executive Officers, Head of Financial Institutions & Public Sector Deputy Head of Risk Management Group Business Unit / Head of Transaction Banking Unit as defined in the Companies Act Kazuhisa Sawa Yasuhiro Sato Tomoshige Jingu Managing Executive Officer Director, President & Group CEO Managing Executive Officer Deputy Head of Strategic Planning Group / Deputy (Representative Executive Officer) Head of Human Resources Group (Group CHRO) Head of Financial Control & Accounting Group / Deputy Head of IT & Systems Group / Deputy Head Yasunori Tsujita of Operations Group Executive Officers, as defined in Director, Deputy President & Executive Officer Takashi Kamada (Representative Executive Officer) our internal regulations Managing Executive Officer Head of Internal Control (Risk Management, Human Deputy Head of Personal Banking Unit / Deputy Resources and Compliance) and Head of Compliance Masaaki Kono Head of Retail Banking Unit Group (Group CCO) Deputy President & Executive Officer Deputy President (Western Japan (Kinki, Chugoku Ryusuke Aya and Shikoku regions)) Yoshihito Bando Managing Executive Officer Director, Managing Executive Officer Deputy Head of Asset Management Unit Head of Risk Management Group (Group CRO) Katsunobu Motohashi Managing Executive Officer Junichi Shinbo Head of Asset Management Unit Kenichi Minamitani Managing Executive Officer Director, Managing Executive Officer Deputy -

Comprehensive List of Pharmaceuticals-Related Projects
Pharmaceutical and R&D Facilities Facility Type Photo Client Location Completion Scope Wakayama/ Solid Dosage TAMURA PHARMACEUTICAL CO.,LTD. 2019 EPCV Japan Gunma/ Bio Bulk DAIICHI SANKYO CHEMICAL PHARMA CO., LTD. 2019 EPCV Japan Hyogo/ R&D Center KANAE CO.,LTD. 2019 EPC Japan API undisclosed Tohoku/Japan 2017 EPCV R&D Center JSR Corporation Tokyo/Japan 2017 EPCV API undisclosed Kitakanto/Japan 2017 EPCV Yamaguchi/ R&D Center TEIJIN PHARMA LIMITED 2015 EPCV Japan Medical Device Menicon Co., Ltd. Chubu/Japan 2014 EPCV Fukushima/ R&D Center SANWA KAGAKU KENKYUSHO CO., LTD. 2014 EPCV Japan Yamaguchi/ Medical Device Terumo Yamaguchi Corporation 2014 E+ES Japan TOYAMA CHEMICAL CO., LTD. Toyama/ Parenteral 2013 EPCV (Current name:FUJIFILM Toyama Chemical Co., Ltd.) Japan Astellas Pharma Inc. Aichi/ Bio Bulk 2013 EPCV (Current name:Micro Biopharm Japan Co.,Ltd.) Japan Kanagawa/ R&D Center Mitsubishi Tanabe Pharma Corporation 2011 EPC Japan Hiroshima/ R&D Center YASUHARA CHEMICAL CO., LTD. 2010 EPC Japan Fukushima/ API TOA EIYO LTD. 2009 EPCV Japan Gunma/ R&D Center SANDEN HOLDINGS CORPORATION 2008 EPC Japan Hyogo/ Packaging KANAE CO., LTD. 2008 EPCV Japan ASAHI GLASS CO., LTD. Chiba/ Bio Bulk 2008 EPCV (Current name:AGC Inc.) Japan 1 / 3 Iwate/ Aseptic API Shionogi & Co., Ltd. 2007 EPCV Japan Mie/ Solid Dosage Sumitomo Dainippon Pharma Co., Ltd. 2007 EPCV Japan Saitama/ Nutrition/Liquid Taisho Pharmaceutical Co., Ltd. 2007 EPCV Japan Kaketsuken Kumamoto/ Vaccine (The Chemo-sero-therapeutic Research Institute) 2006 EPCV Japan (Current name:KM Biologics Co., Ltd.) Niigata/ Vaccine Denka Seiken Co., Ltd. 2006 EPCV Japan Shizuoka/ Bio Bulk Yamaha Motor Co., Ltd. -

TSHP Virtual Seminar 2020 Partner, Supporter, and Exhibitor Listing
TSHP Virtual Seminar 2020 Partner, Supporter, and Exhibitor Listing Abbvie https://www.abbvie.com/ ___________________________________________________________________________________ AcelRx Pharmaceuticals http://www.acelrx.com AcelRx Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on the development and commercialization of innovative therapies for use in medically supervised settings. In November of 2018, its lead product candidate DSUVIA™ (sufentanil sublingual tablet 30 mcg) was approved by the U.S. Food and Drug Administration. ___________________________________________________________________________________ AIS Healthcare http://www.aiscaregroup.com The leading 503A specialty pharmacy and targeted drug delivery care provider, AIS Healthcare is driven to improve the everyday lives of patients and providers, in ways big and small. From higher quality and sterility standards to services that improve care delivery, we do what’s right, not just what’s expected— every time. ___________________________________________________________________________________ Allergan http://www.allergan.com Allergan is a world leader in neuromodulator therapy and neurosciences. For over three decades, we have been committed to the research and clinical development of BOTOX® (OnabotulinumtoxinA), one of the world’s most versatile medicines, to improve the physical well-being and resulting quality of life for people around the world who suffer from a variety of serious or debilitating disorders. BOTOX® is available in more than 75 countries with 20 approved indications. ___________________________________________________________________________________ AMAG Pharmaceuticals http://www.amagpharma.com AMAG is a pharmaceutical company focused on bringing innovative products to patients with unmet medical needs. Every day the people at AMAG aim higher, devoting our passion and perseverance to finding new and better ways to support the health of patients and families. For additional company information, please visit www.amagpharma.com. -

Press Release New AMR Action Fund Steps in to Save Collapsing Antibiotic Pipeline with Pharmaceutical Industry Investment of US$1 Billion
Press Release New AMR Action Fund steps in to save collapsing antibiotic pipeline with pharmaceutical industry investment of US$1 billion - Partnership aims to bring 2 to 4 new antibiotics to patients by the end of the decade and facilitate needed long-term policy solutions - OSAKA, Japan, July 10, 2020 — Today, more than 20 leading biopharmaceutical companies including Shionogi & Co., Ltd. (Head Office: Chuo-ku, Osaka, Japan; President & CEO: Isao Teshirogi, Ph.D.; hereinafter, “Shionogi” or the “Company”) announced the launch of the AMR Action Fund [www.AMRactionfund.com], a ground-breaking partnership that aims to bring 2-4 new antibiotics to patients by 2030. These treatments are urgently needed to address the rapid rise of antibiotic-resistant infections – also called antimicrobial resistance, or AMR. The companies have raised so far nearly US$1 billion new funding to support clinical research of innovative new antibiotics that are addressing the most resistant bacteria and life-threatening infections, and Shionogi will invest US$20 million. Through the AMR Action Fund, pharmaceutical companies will join forces with philanthropies, development banks, and multilateral organizations to strengthen and accelerate antibiotic development. The Fund will focus on urgent public health needs. It will provide much needed financial resources, as well as important technical support to help biotech companies bring novel antibiotics to patients. The AMR Action Fund, an initiative of the international body representing the R&D pharmaceutical industry (International Federation of Pharmaceutical Manufacturers & Associations, IFPMA), was announced at simultaneous virtual launch events in Berlin, Germany, and Washington, D.C., USA, with a third event in Tokyo, Japan taking place on July 10. -

59317809.Pdf
171 171 BUSINESS REVIEW Vo l. 61. No. 4 March. March. 2014 How to Balance the Intellectual Property Conflic t: a Case Study of J apanese Pharmaceutical Companies in Emerging Countries YuJn-r-mw XMum 一明 MPν νゎ J'ιY Abstract Abstract Pharmaceutical companies entering emerging countries wiU face intellectual property property conflicts due to issues relating to thc access to medicines of consumcrs in those countnes. “Intellectual property conflic t" refers to the conflict of intercst which exists between manufacturers ,who own intellectual property , and consumers ,who do no t. In In reviewing the representative literature , there is a focus on the pricing patterns (known as differential pricing) of pharmaceuticals. This is a way of setting different prices for the the same products in different areas or countries , in particular setting a low price for low- income income consumers. while at the same time maintaining the pharmaceutical companies' profits by granting patents. In In spite of the growing literature on differential pricing , research in the field of corporate corporate behavior reviewed has been relatively lcss dctailed. This paper explores the issue issue from a corporate perspective , by examining four R&D-driven PCs in J apan: Takeda Pharmaceutical Pharmaceutical Company Limited (Takeda) , Daiichi Sankyo Company Limited (Daiichi Sankyo) ,Eisai Co. ,L TD (E isai) and Astellas Pharma Inc. (Astellas) , mainly through the use of of quantitative data as a means to determine key financial indicators during the period 20 ∞- 2013 2013 reviewed 172 172 一一- BUSINESS REVIEW -一一 Keywords: Intellectual Property (IP) ,Intellectual Property Conflict (IP Conflict) ,Paten t, Pharmaceutical Pharmaceutical Companies (PCs) ,Emerging Countries , Access to Medicines 1.