View a Copy of This Licence, Visit Tivecommons.Org/Licenses/By/4.0
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Material
Supplementary material Table S1. Search strategy performed on the following databases: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL). 1. Randomi*ed study OR random allocation OR Randomi*ed controlled trial OR Random* Control* trial OR RCT Epidemiological study 2. sodium glucose cotransporter 2 OR sodium glucose cotransporter 2 inhibitor* OR sglt2 inhibitor* OR empagliflozin OR dapagliflozin OR canagliflozin OR ipragliflozin OR tofogliflozin OR ertugliflozin OR sotagliflozin OR sergliflozin OR remogliflozin 3. 1 AND 2 1 Table S2. Safety outcomes of empagliflozin and linagliptin combination therapy compared with empagliflozin or linagliptin monotherapy in treatment naïve type 2 diabetes patients Safety outcome Comparator 1 Comparator 2 I2 RR [95% CI] Number of events Number of events / / total subjects total subjects i. Empagliflozin + linagliptin vs empagliflozin monotherapy Empagliflozin + Empagliflozin linagliptin monotherapy ≥ 1 AE(s) 202/272 203/270 77% 0.99 [0.81, 1.21] ≥ 1 drug-related 37/272 38/270 0% 0.97 [0.64, 1.47] AE(s) ≥ 1 serious AE(s) 13/272 19/270 0% 0.68 [0.34, 1.35] Hypoglycaemia* 0/272 5/270 0% 0.18 [0.02, 1.56] UTI 32/272 25/270 29% 1.28 [0.70, 2.35] Events suggestive 12/272 13/270 9% 0.92 [0.40, 2.09] of genital infection i. Empagliflozin + linagliptin vs linagliptin monotherapy Empagliflozin + Linagliptin linagliptin monotherapy ≥ 1 AE(s) 202/272 97/135 0% 1.03 [0.91, 1.17] ≥ 1 drug-related 37/272 17/135 0% 1.08 [0.63, 1.84] AE(s) ≥ 1 serious AE(s) 13/272 2/135 0% 3.22 [0.74, 14.07] Hypoglycaemia* 0/272 1/135 NA 0.17 [0.01, 4.07] UTI 32/272 12/135 0% 1.32 [0.70, 2.49] Events suggestive 12/272 4/135 0% 1.45 [0.47, 4.47] of genital infection RR, relative risk; AE, adverse event; UTI, urinary tract infection. -

Glucose Cotransporter 2 Inhibitor, Attenuates Body Weight Gain and Fat Accumulation in Diabetic and Obese Animal Models
OPEN Citation: Nutrition & Diabetes (2014) 4, e125; doi:10.1038/nutd.2014.20 & 2014 Macmillan Publishers Limited All rights reserved 2044-4052/14 www.nature.com/nutd ORIGINAL ARTICLE Tofogliflozin, a sodium/glucose cotransporter 2 inhibitor, attenuates body weight gain and fat accumulation in diabetic and obese animal models M Suzuki1, M Takeda1, A Kito1, M Fukazawa1, T Yata2, M Yamamoto1, T Nagata1, T Fukuzawa1, M Yamane1, K Honda1, Y Suzuki1 and Y Kawabe1 OBJECTIVE: Tofogliflozin, a highly selective inhibitor of sodium/glucose cotransporter 2 (SGLT2), induces urinary glucose excretion (UGE), improves hyperglycemia and reduces body weight in patients with Type 2 diabetes (T2D). The mechanisms of tofogliflozin on body weight reduction were investigated in detail with obese and diabetic animal models. METHODS: Diet-induced obese (DIO) rats and KKAy mice (a mouse model of diabetes with obesity) were fed diets containing tofogliflozin. Body weight, body composition, biochemical parameters and metabolic parameters were evaluated. RESULTS: In DIO rats tofogliflozin was administered for 9 weeks, UGE was induced and body weight gain was attenuated. Body fat mass decreased without significant change in bone mass or lean body mass. Food consumption (FC) increased without change in energy expenditure, and deduced total calorie balance (deduced total calorie balance ¼ FC À UGE À energy expenditure) decreased. Respiratory quotient (RQ) and plasma triglyceride (TG) level decreased, and plasma total ketone body (TKB) level increased. Moreover, plasma leptin level, adipocyte cell size and proportion of CD68-positive cells in mesenteric adipose tissue decreased. In KKAy mice, tofogliflozin was administered for 3 or 5 weeks, plasma glucose level and body weight gain decreased together with a reduction in liver weight and TG content without a reduction in body water content. -

Comparison of Tofogliflozin 20 Mg and Ipragliflozin 50 Mg Used Together
2017, 64 (10), 995-1005 Original Comparison of tofogliflozin 20 mg and ipragliflozin 50 mg used together with insulin glargine 300 U/mL using continuous glucose monitoring (CGM): A randomized crossover study Soichi Takeishi, Hiroki Tsuboi and Shodo Takekoshi Department of Diabetes, General Inuyama Chuo Hospital, Inuyama 484-8511, Japan Abstract. To investigate whether sodium glucose co-transporter 2 inhibitors (SGLT2i), tofogliflozin or ipragliflozin, achieve optimal glycemic variability, when used together with insulin glargine 300 U/mL (Glargine 300). Thirty patients with type 2 diabetes were randomly allocated to 2 groups. For the first group: After admission, tofogliflozin 20 mg was administered; Fasting plasma glucose (FPG) levels were titrated using an algorithm and stabilized at 80 mg/dL level with Glargine 300 for 5 days; Next, glucose levels were continuously monitored for 2 days using continuous glucose monitoring (CGM); Tofogliflozin was then washed out over 5 days; Subsequently, ipragliflozin 50 mg was administered; FPG levels were titrated using the same algorithm and stabilized at 80 mg/dL level with Glargine 300 for 5 days; Next, glucose levels were continuously monitored for 2 days using CGM. For the second group, ipragliflozin was administered prior to tofogliflozin, and the same regimen was maintained. Glargine 300 and SGLT2i were administered at 8:00 AM. Data collected on the second day of measurement (mean amplitude of glycemic excursion [MAGE], average daily risk range [ADRR]; on all days of measurement) were analyzed. Area over the glucose curve (<70 mg/dL; 0:00 to 6:00, 24-h), M value, standard deviation, MAGE, ADRR, and mean glucose levels (24-h, 8:00 to 24:00) were significantly lower in patients on tofogliflozin than in those on ipragliflozin. -

Ranking of Stocks by Market Capitalization(As of End of Mar.2020)
Ranking of Stocks by Market Capitalization(As of End of Mar.2020) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 212,127 2 9437 NTT DOCOMO,INC. 112,630 3 6861 KEYENCE CORPORATION 84,709 4 6758 SONY CORPORATION 81,786 5 9984 SoftBank Group Corp. 79,162 6 9433 KDDI CORPORATION 75,136 7 4519 CHUGAI PHARMACEUTICAL CO.,LTD. 69,960 8 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 68,006 9 9434 SoftBank Corp. 65,799 10 7974 Nintendo Co.,Ltd. 54,787 11 8306 Mitsubishi UFJ Financial Group,Inc. 54,735 12 4568 DAIICHI SANKYO COMPANY,LIMITED 52,707 13 4502 Takeda Pharmaceutical Company Limited 52,146 14 4661 ORIENTAL LAND CO.,LTD. 50,261 15 6098 Recruit Holdings Co.,Ltd. 47,419 16 9983 FAST RETAILING CO.,LTD. 46,873 17 7182 JAPAN POST BANK Co.,Ltd. 44,865 18 4063 Shin-Etsu Chemical Co.,Ltd. 44,707 19 7267 HONDA MOTOR CO.,LTD. 44,017 20 4452 Kao Corporation 42,560 21 6367 DAIKIN INDUSTRIES,LTD. 38,603 22 6981 Murata Manufacturing Co.,Ltd. 36,980 23 8058 Mitsubishi Corporation 36,436 24 8316 Sumitomo Mitsui Financial Group,Inc. 36,018 25 9022 Central Japan Railway Company 35,679 26 8001 ITOCHU Corporation 35,541 27 8766 Tokio Marine Holdings,Inc. 35,145 28 7741 HOYA CORPORATION 34,808 29 6594 NIDEC CORPORATION 33,433 30 8035 Tokyo Electron Limited 32,000 31 3382 Seven & I Holdings Co.,Ltd. 31,699 32 7751 CANON INC. 31,463 33 8411 Mizuho Financial Group,Inc. -

Populationsweite Utilisationsuntersuchung in Den Chronischen Krankheitsbildern Hypertonie, Hyperlipid¨Amieund Typ 2 Diabetes Mellitus –
Ruhr-Universit¨atBochum Prof. Dr. rer. nat. Hans J. Trampisch Dienstort: Abteilung f¨urMedizinische Informatik, Biometrie und Epidemiologie Populationsweite Utilisationsuntersuchung in den chronischen Krankheitsbildern Hypertonie, Hyperlipid¨amieund Typ 2 Diabetes Mellitus { Eine Studie des Projekts PUKO-BHD an der Medizinischen Universit¨atWien Inaugural-Dissertation zur Erlangung des Doktorgrades der Medizin einer Hohen Medizinischen Fakult¨at der Ruhr-Universit¨atBochum vorgelegt von: Lisanne M. Jandeck aus Herford 2014 Dekan: Prof. Dr. med. Albrecht Bufe Referent: Prof. Dr. rer. nat. Hans J. Trampisch Korreferent: Prof. Dr. med. J¨urgenWindeler Tag der M¨undlichen Pr¨ufung:09.02.2017 Abstract Jandeck Lisanne M. Populationsweite Utilisationsuntersuchung in den chronischen Krankheitsbildern Hypertonie, Hyperlipid¨amieund Typ 2 Diabetes Mellitus Problem: Hypertonie (HT), Hyperlipid¨amie(HL) und Typ 2 Diabetes Mellitus (DM) stellen die h¨aufigstenchronischen Erkrankungen in Osterreich¨ dar, besonders bei Uber-50j¨ahrigen.Die¨ Zielsetzung dieser Teilstudie Utilisation\ des Projekts mit " dem Titel PUKO-BHD { Pr¨avalenz, Utilisation, Kosten und Outcome bei Blut- " hochdruck, Hyperlipid¨amieund Typ 2 Diabetes Mellitus\ ist eine populationsweite epidemiologische Untersuchung zu der Utilisation von Arzneimitteln zur Behand- lung dieser Krankheiten, insbesondere in Bezug auf Mehrfachverschreibungen (dou- ble prescriptions, DP) und Verschreibungen von Kombinationen von Wirkstoffen, die (schwerwiegende) Wechselwirkungen erzeugen k¨onnen (drug-drug -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -

Clinical Comparison of Tofogliflozin and Empagliflozin Based on An
Obesity Medicine 14 (2019) 100088 Contents lists available at ScienceDirect Obesity Medicine journal homepage: www.elsevier.com/locate/obmed Original research Clinical comparison of tofogliflozin and empagliflozin based on an analysis of 24-h accumulated urine in Japanese patients with type 2 diabetes mellitus T ∗ Kazuo Kobayashia, , Masao Toyodab, Nobuo Hatoric a Kobayashi Clinic of Internal Medicine, Sagamihara, Japan b Division of Nephrology, Endocrinology and Metabolism, Department of Internal Medicine, Tokai University School of Medicine, Isehara, Japan c Kobayashi Hospital, Odawara, Japan ARTICLE INFO ABSTRACT Keywords: Aim: In Japan, six sodium-glucose co-transporter 2 inhibitors have been approved for use, and some agents are Tofogliflozin associated with a significant decrease in cardiovascular events. In this study, the effects of tofogliflozin and Empagliflozin empagliflozin were compared. 24-H accumulated urine Methods: Patients with type 2 diabetes mellitus who were administered tofogliflozin (n = 10) and empagliflozin Sodium-glucose co-transporter 2 inhibitor (n = 12) were extracted. The clinical parameters and 24-h accumulated urine samples before and after 48 weeks Hematocrit were analyzed with generalized linear mixed model. Results: Both groups showed significant differences in the following parameters: body weight (p < 0.0001), mean blood pressure (p = 0.006), glycated hemoglobinA1c (p < 0.0001), alanine aminotransferase (p < 0.0001), high-density lipoprotein cholesterol (p < 0.0001), homeostatic model assessment 2 (%S) (p = 0.006), volume of 24-h accumulated urine (p < 0.0001), and 24-h urine glucose excretion (p < 0.0001). The hematocrit differed significantly over the study period (p < 0.0001); however, empaglifozin had a sig- nificantly stronger effect on the hematocrit than tofoglifozin (p = 0.007). -
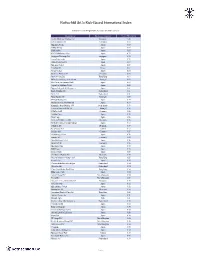
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Association Between Variability in Body Mass Index and Development of Type 2 Diabetes: Panasonic Cohort Study
Epidemiology/Health services research Open access Original research BMJ Open Diab Res Care: first published as 10.1136/bmjdrc-2021-002123 on 22 April 2021. Downloaded from Association between variability in body mass index and development of type 2 diabetes: Panasonic cohort study Hiroshi Okada ,1 Masahide Hamaguchi ,2 Momoko Habu,1 Kazushiro Kurogi,3 Hiroaki Murata,4 Masato Ito,3 Michiaki Fukui2 To cite: Okada H, ABSTRACT Hamaguchi M, Habu M, Introduction Contrasting results have been reported for Significance of this study et al. Association between the association between the variability in body weight variability in body mass index and development of diabetes. In the present study, we What is already known about this subject? and development of type 2 evaluated the association between the variability in body ► Obesity or body weight gain accelerate the develop- diabetes: Panasonic cohort ment of diabetes. study. BMJ Open Diab Res Care mass index (BMI) and development of type 2 diabetes in 19 412 Japanese participants without obesity and without ► The association between the variability in body 2021;9:e002123. doi:10.1136/ weight and development of diabetes is controversial. bmjdrc-2021-002123 body weight gain or loss during the study period. Research design and methods We recorded body weight What are the new findings? of the participants consecutively each year in Panasonic ► The risk for developing diabetes increased by 11.1% Received 9 January 2021 Corporation, Osaka, Japan from 2008 to 2014 to evaluate Revised 26 February 2021 per 1% increase in coefficient of variation of body the variability of BMI. The participants with obesity (BMI mass index (BMI). -

Effect of Tofogliflozin on Arterial Stiffness in Patients with Type 2
Katakami et al. Cardiovasc Diabetol (2021) 20:4 https://doi.org/10.1186/s12933-020-01206-1 Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Efect of tofoglifozin on arterial stifness in patients with type 2 diabetes: prespecifed sub-analysis of the prospective, randomized, open-label, parallel-group comparative UTOPIA trial Naoto Katakami1,2* , Tomoya Mita3, Hidenori Yoshii4, Toshihiko Shiraiwa5, Tetsuyuki Yasuda6, Yosuke Okada7, Keiichi Torimoto7, Yutaka Umayahara8, Hideaki Kaneto9, Takeshi Osonoi10, Tsunehiko Yamamoto11, Nobuichi Kuribayashi12, Kazuhisa Maeda13, Hiroki Yokoyama14, Keisuke Kosugi15, Kentaro Ohtoshi16, Isao Hayashi17, Satoru Sumitani18, Mamiko Tsugawa19, Kayoko Ryomoto20, Hideki Taki21, Tadashi Nakamura22, Satoshi Kawashima23, Yasunori Sato24, Hirotaka Watada3, Iichiro Shimomura1 and On behalf of the UTOPIA study investigators Abstract Background: Tofoglifozin, an SGLT2 inhibitor, is associated with favorable metabolic efects, including improved glycemic control and serum lipid profle and decreased body weight, visceral adipose tissue, and blood pressure (BP). This study evaluated the efects of tofoglifozin on the brachial-ankle pulse wave velocity (baPWV) in patients with type 2 diabetes (T2DM) without a history of apparent cardiovascular disease. Methods: The using tofoglifozin for possible better intervention against atherosclerosis for type 2 diabetes patients (UTOPIA) trial is a prospective, randomized, open-label, multicenter, parallel-group, comparative study. As one of the prespecifed secondary outcomes, -

Identification of SGLT2 Inhibitor Ertugliflozin As a Treatment
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.18.448921; this version posted June 18, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Identification of SGLT2 inhibitor Ertugliflozin as a treatment for COVID-19 using computational and experimental paradigm Shalini SaxenaA, Kranti MeherC, Madhuri RotellaC, Subhramanyam VangalaC, Satish ChandranC, Nikhil MalhotraB, Ratnakar Palakodeti B, Sreedhara R Voleti A*, and Uday SaxenaC* A In Silico Discovery Research Academic Services (INDRAS) Pvt. Ltd. 44-347/6, Tirumalanagar, Moula Ali, Hyderabad – 500040, TS, India B Tech Mahindra Gateway Building, Apollo Bunder, Mumbai-400001, Maharashtra, India C Reagene Innovation Pvt. Ltd. 18B, ASPIRE-BioNEST, 3rd Floor, School of Life Sciences, University of Hyderabad, Gachibowli, Hyderabad – 500046, TS, India *Corresponding Authors email address: [email protected] [email protected] Abstract Drug repurposing can expedite the process of drug development by identifying known drugs which are effective against SARS-CoV-2. The RBD domain of SARS-CoV-2 Spike protein is a promising drug target due to its pivotal role in viral-host attachment. These specific structural domains can be targeted with small molecules or drug to disrupt the viral attachment to the host proteins. In this study, FDA approved Drugbank database were screened using a virtual screening approach and computational chemistry methods. Five drugs were short listed for further profiling based on docking score and binding energies. Further these selected drugs were tested for their in vitro biological activity. -

Notice of the Ordinary General Meeting of Shareholders to Be Held on June 26, 2020
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 26, 2020 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 26, 2020 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To amend a part of the Articles of Incorporation. 2. To elect 12 Directors. 3. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020). Note: The Consolidated Financial Statements pursuant to the Companies Act of Japan (Translation) are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting103/ 1 PROPOSALS TO BE ACTED UPON: 1.