Association Between Variability in Body Mass Index and Development of Type 2 Diabetes: Panasonic Cohort Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View a Copy of This Licence, Visit Tivecommons.Org/Licenses/By/4.0
Katakami et al. Cardiovasc Diabetol (2020) 19:110 https://doi.org/10.1186/s12933-020-01079-4 Cardiovascular Diabetology ORIGINAL INVESTIGATION Open Access Tofoglifozin does not delay progression of carotid atherosclerosis in patients with type 2 diabetes: a prospective, randomized, open-label, parallel-group comparative study Naoto Katakami1,2* , Tomoya Mita3, Hidenori Yoshii4, Toshihiko Shiraiwa5, Tetsuyuki Yasuda6, Yosuke Okada7, Keiichi Torimoto7, Yutaka Umayahara8, Hideaki Kaneto9, Takeshi Osonoi10, Tsunehiko Yamamoto11, Nobuichi Kuribayashi12, Kazuhisa Maeda13, Hiroki Yokoyama14, Keisuke Kosugi15, Kentaro Ohtoshi16, Isao Hayashi17, Satoru Sumitani18, Mamiko Tsugawa19, Kayoko Ryomoto20, Hideki Taki21, Tadashi Nakamura22, Satoshi Kawashima23, Yasunori Sato24, Hirotaka Watada3 and Iichiro Shimomura1 on behalf of the UTOPIA study investigators Abstract Background: This study aimed to investigate the preventive efects of tofoglifozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor, on atherosclerosis progression in type 2 diabetes (T2DM) patients without apparent cardiovascular disease (CVD) by monitoring carotid intima-media thickness (IMT). Methods: This prospective, randomized, open-label, blinded-endpoint, multicenter, parallel-group, comparative study included 340 subjects with T2DM and no history of apparent CVD recruited at 24 clinical units. Subjects were randomly allocated to either the tofoglifozin treatment group (n 169) or conventional treatment group using drugs other than SGLT2 inhibitors (n 171). Primary outcomes were changes= in mean and maximum common carotid IMT measured by echography during= a 104-week treatment period. Results: In a mixed-efects model for repeated measures, the mean IMT of the common carotid artery (mean- IMT-CCA), along with the right and left maximum IMT of the CCA (max-IMT-CCA), signifcantly declined in both the tofoglifozin ( 0.132 mm, SE 0.007; 0.163 mm, SE 0.013; 0.170 mm, SE 0.020, respectively) and the control group ( 0.140 mm,− SE 0.006; 0.190 mm,− SE 0.012; 0.190 mm,− SE 0.020, respectively). -

Ranking of Stocks by Market Capitalization(As of End of Mar.2020)
Ranking of Stocks by Market Capitalization(As of End of Mar.2020) 1st Section Rank Code Issue Market Capitalization \100mil. 1 7203 TOYOTA MOTOR CORPORATION 212,127 2 9437 NTT DOCOMO,INC. 112,630 3 6861 KEYENCE CORPORATION 84,709 4 6758 SONY CORPORATION 81,786 5 9984 SoftBank Group Corp. 79,162 6 9433 KDDI CORPORATION 75,136 7 4519 CHUGAI PHARMACEUTICAL CO.,LTD. 69,960 8 9432 NIPPON TELEGRAPH AND TELEPHONE CORPORATION 68,006 9 9434 SoftBank Corp. 65,799 10 7974 Nintendo Co.,Ltd. 54,787 11 8306 Mitsubishi UFJ Financial Group,Inc. 54,735 12 4568 DAIICHI SANKYO COMPANY,LIMITED 52,707 13 4502 Takeda Pharmaceutical Company Limited 52,146 14 4661 ORIENTAL LAND CO.,LTD. 50,261 15 6098 Recruit Holdings Co.,Ltd. 47,419 16 9983 FAST RETAILING CO.,LTD. 46,873 17 7182 JAPAN POST BANK Co.,Ltd. 44,865 18 4063 Shin-Etsu Chemical Co.,Ltd. 44,707 19 7267 HONDA MOTOR CO.,LTD. 44,017 20 4452 Kao Corporation 42,560 21 6367 DAIKIN INDUSTRIES,LTD. 38,603 22 6981 Murata Manufacturing Co.,Ltd. 36,980 23 8058 Mitsubishi Corporation 36,436 24 8316 Sumitomo Mitsui Financial Group,Inc. 36,018 25 9022 Central Japan Railway Company 35,679 26 8001 ITOCHU Corporation 35,541 27 8766 Tokio Marine Holdings,Inc. 35,145 28 7741 HOYA CORPORATION 34,808 29 6594 NIDEC CORPORATION 33,433 30 8035 Tokyo Electron Limited 32,000 31 3382 Seven & I Holdings Co.,Ltd. 31,699 32 7751 CANON INC. 31,463 33 8411 Mizuho Financial Group,Inc. -

Press Release
Press Release Company name: DAIICHI SANKYO COMPANY, LIMITED Representative: Sunao Manabe, Representative Director, President and CEO (Code no.: 4568, First Section, Tokyo Stock Exchange) Please address inquiries to Junichi Onuma, Vice President, Corporate Communications Department Telephone: +81-3-6225-1126 https://www.daiichisankyo.com Daiichi Sankyo Announces Transfer from Astellas Pharma of Three Products in Asia Tokyo, Japan (October 15, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) today announced that it agreed with Astellas Pharma Inc. (hereafter, Astellas Pharma) that Astellas Pharma local subsidiaries companies in six Asian countries will transfer three products to Daiichi Sankyo. The products to be transferred and the countries where they are sold are as follows. Product Korea China Taiwan Thailand Philippines Indonesia [generic name (brand name)] Ramosetron Antiemetic ○ ○ ○ ○ (Nasea) Nicardipine Anti- ○ ○ ○ (Perdipine) hypertensive Barnidipine Anti- ○ (Oldeca) hypertensive ○ indicate the countries where the products are sold The antiemetics are expected to have synergistic effects with mirogabalin and the cancer drugs that Daiichi Sankyo is currently developing in Asia, and the two antihypertensives are expected to effectively utilize Daiichi Sankyo’s current infrastructures in combination with its cardiovascular products, such as olmesartan and edoxaban. The total net sales of Astellas Pharma's three products in fiscal year 2018 were approximately 5.0 billion yen. 1 Daiichi Sankyo will take over the rights -
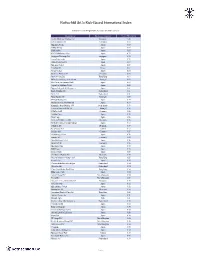
R&Co Risk-Based International Index – Weighting
Rothschild & Co Risk-Based International Index Indicative Index Weight Data as of June 30, 2021 on close Constituent Exchange Country Index Weight(%) Jardine Matheson Holdings Ltd Singapore 1.46 LEG Immobilien SE Germany 0.98 Ajinomoto Co Inc Japan 0.95 SoftBank Corp Japan 0.89 Shimano Inc Japan 0.85 FUJIFILM Holdings Corp Japan 0.73 Singapore Exchange Ltd Singapore 0.72 Japan Tobacco Inc Japan 0.72 Cellnex Telecom SA Spain 0.69 Nintendo Co Ltd Japan 0.69 Carrefour SA France 0.67 Nexon Co Ltd Japan 0.66 Deutsche Wohnen SE Germany 0.65 Bank of China Ltd Hong Kong 0.64 REN - Redes Energeticas Nacion Portugal 0.63 Pan Pacific International Hold Japan 0.63 Japan Post Holdings Co Ltd Japan 0.62 Nippon Telegraph & Telephone C Japan 0.61 Roche Holding AG Switzerland 0.61 Nestle SA Switzerland 0.61 Novo Nordisk A/S Denmark 0.59 ENEOS Holdings Inc Japan 0.59 Nomura Research Institute Ltd Japan 0.59 Koninklijke Ahold Delhaize NV Netherlands 0.59 Jeronimo Martins SGPS SA Portugal 0.58 HelloFresh SE Germany 0.58 Toshiba Corp Japan 0.58 Hoya Corp Japan 0.58 Siemens Healthineers AG Germany 0.58 MS&AD Insurance Group Holdings Japan 0.57 Coloplast A/S Denmark 0.57 Kerry Group PLC Ireland 0.57 Scout24 AG Germany 0.57 SG Holdings Co Ltd Japan 0.56 Symrise AG Germany 0.56 Nitori Holdings Co Ltd Japan 0.56 Beiersdorf AG Germany 0.55 Mitsubishi Corp Japan 0.55 KDDI Corp Japan 0.55 Sysmex Corp Japan 0.55 Chr Hansen Holding A/S Denmark 0.55 Ping An Insurance Group Co of Hong Kong 0.55 Eisai Co Ltd Japan 0.54 Chocoladefabriken Lindt & Spru Switzerland 0.54 Givaudan -

Notice of the Ordinary General Meeting of Shareholders to Be Held on June 26, 2020
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 26, 2020 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 26, 2020 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To amend a part of the Articles of Incorporation. 2. To elect 12 Directors. 3. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2020 (from April 1, 2019 to March 31, 2020). Note: The Consolidated Financial Statements pursuant to the Companies Act of Japan (Translation) are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting103/ 1 PROPOSALS TO BE ACTED UPON: 1. -

Astellas Pharma Inc. 2-5-1, Nihonbashi-Honcho, Chuo-Ku, Tokyo 103-8411, Japan Astellas Pharma Inc
ANNUAL REPORT 2016 ANNUAL REPORT 2016 For the Year Ended March 31, 2016 Astellas Pharma Inc. 2-5-1, Nihonbashi-Honcho, Chuo-ku, Tokyo 103-8411, Japan http://www.astellas.com/en/ Astellas Pharma Inc. Please direct inquiries concerning Annual Report 2016 to: Astellas Pharma Inc. Corporate Communications TEL: +81-3-3244-3202 FAX: +81-3-5201-7473 This report is printed with environmentally friendly vegetable-based inks on FSCTM-certified paper made of wood sourced from responsibly managed forests, Issued in August 2016 using a waterless printing process. Printed in Japan Contents About Astellas 1 About Astellas 2 Business Philosophy Retracing Astellas’ Steps 3 Astellas Today 5 Contribute toward Health Financial and Non-Financial Highlights 7 Raison D'être Editorial Policy 9 Contribute toward improving the health of people through Innovative Drugs around the world through the provision of innovative 2 Our Strategy 10 and reliable pharmaceutical products 1 Astellas Value Creation Process 11 • To go beyond all others in exploring and tapping the potential CEO Message Astellas is concentrating on the 13 of the life sciences. CSR-Based Management 17 innovative drug business, where Business Environment 19 • To continue tackling new challenges and creating innovative it possesses key strengths. Strategic Plan 2015-2017 pharmaceutical products. 20 We are enhancing our ability to Financial Strategy 24 • To deliver quality products along with accurate information Management Structure 25 and retain solid credibility among customers. continuously develop innovative Interview with an Outside Director 27 drugs, in conjunction with • To support healthy living for people around the world. optimizing the allocation of • To continue shining on the global pharmaceutical field. -

INTA Corporate Member List
Corporate Members Name City State Country 100x Group Hong Kong Hong Kong 1661, Inc. Los Angeles California United States 1-800-Flowers.com, Inc. Carle Place New York United States 3M China Ltd. Shanghai Shanghai China 3M Company Saint Paul Minnesota United States 3M Deutschland GmbH Neuss Germany 3M India Ltd. Bangalore Karnataka India 3M Innovation Singapore Pte Ltd Singapore Singapore 3M KCI San Antonio Texas United States 3M United Kingdom PLC Bracknell Bracknell Forest United Kingdom 7-Eleven, Inc. Irving Texas United States AB Electrolux Stockholm Stockholms län Sweden Västra Götalands AB Volvo Göteborg län Sweden ABB ASEA Brown Boveri Ltd. Zürich Zürich Switzerland Abbott St.Paul Minnesota United States Abbott Laboratories Abbott Park Illinois United States Abbott Products Operations AG Allschwil Switzerland AbbVie Inc. Irvine California United States AbbVie Inc. North Chicago Illinois United States Abercrombie & Fitch Co. New Albany Ohio United States ABRO Industries, Inc. South Bend Indiana United States ACCO Brands Corporation Lake Zurich Illinois United States Activision Blizzard UK Ltd. Slough United Kingdom Activision Blizzard, Inc. Santa Monica California United States Activision Computer Technology Co. Ltd. Shanghai Shanghai China Acushnet Company Fairhaven Massachusetts United States Aderans America Holdings, Inc. Beverly Hills California United States adidas Group Panama Panamá Panama Adidas Group, India Gurgaon Haryana India adidas International Marketing BV Amsterdam Netherlands adidas International, Inc. Portland Oregon United States ADOBE INC. San Jose California United States ADOBE INC. Tokyo Japan Aetna Inc. Hartford Connecticut United States Agilent Technologies, Inc. Santa Clara California United States Ahold Delhaize Licensing Sarl Genève Genève Switzerland AIDA Cruises Rostock Germany Aigle International S.A. -

Astellas Pharma US, Inc. Corporate Integrity Agreement
CORPORATE INTEGRITY AGREEMENT BETWEEN THE OFFICE OF INSPECTOR GENERAL OF THE DEPARTMENT OF HEALTH AND HUMAN SERVICES AND ASTELLAS PHARMA US, INC. I. PREAMBLE Astellas Pharma US Inc. (Astellas) hereby enters into this Corporate Integrity Agreement (CIA) with the Office of Inspector General (OIG) of the United States Department of Health and Human Services (HHS) to promote compliance with the statutes, regulations, and written directives of Medicare, Medicaid, and all other Federal health care programs (as defined in 42 U.S.C. § 1320a-7b(f)) (Federal health care program requirements). Contemporaneously with this CIA, Astellas is entering into a Settlement Agreement with the United States. Prior to the Effective Date, Astellas established a compliance program that Astellas represents addresses all seven elements of an effective compliance program and that is designed to address compliance with Federal health care program requirements (Compliance Program). Astellas shall continue the Compliance Program throughout the term of the CIA and shall do so in accordance with the terms set forth below. Astellas may modify the Compliance Program as appropriate. However, at a minimum, Astellas shall ensure that during the term of this CIA, it shall maintain a compliance program to comply with the obligations set forth in this CIA. II. TERM AND SCOPE OF THE CIA A. The period of the compliance obligations assumed by Astellas under this CIA shall be five years from the effective date of this CIA. The “Effective Date” shall be the date on which the final signatory of this CIA executes this CIA. Each one-year period, beginning with the one-year period following the Effective Date, shall be referred to as a “Reporting Period.” Corporate Integrity Agreement Astellas Pharma US, Inc. -

Holdings KL Allocation Fund, As of 3/31/20
Holdings KL Allocation Fund, as of 3/31/20 Equity Securities % of Net Assets Country Sector CHUGAI PHARM CO 2.33 Japan Health Care FISHER & PAYKEL HE 1.79 New Zealand Health Care L'OREAL 1.72 France Consumer Staples COLOPLAST A/S 1.53 Denmark Health Care KONINKLIJKE DSM NV 1.44 Netherlands Materials INTEL CORP COM 1.13 United States Information Technology STERICYCLE INC 1.08 United States Industrials KAO CORP 1.08 Japan Consumer Staples SMC CORP 1.08 Japan Industrials UNI-CHARM CORP. 1.08 Japan Consumer Staples BIO-TECHNE CORP 1.07 United States Health Care DAIICHI SANKYO COM 1.06 Japan Health Care NOVARTIS AG 1.05 Switzerland Health Care GENTEX CORP 1.05 United States Consumer Discretionary JOHNSON & JOHNSON 1.04 United States Health Care 3M CO COM 1.04 United States Industrials SHIMADZU CORP 1.04 Japan Information Technology ALPHABET INC CAP STK CL A 1.04 United States Communication Services MERCK & CO INC COM 1.03 United States Health Care HISAMITSU PHARM CO 1.03 Japan Health Care PROCTER AND GAMBLE CO COM 1.03 United States Consumer Staples NISSIN FOOD HOLDINGS CO LTD 1.02 Japan Consumer Staples IROBOT CORP 1.01 United States Consumer Discretionary DAIKIN INDUSTRIES LTD 1.01 Japan Industrials NTT DOCOMO 1.00 Japan Communication Services GRIFOLS SA 1.00 Spain Health Care AIR LIQUIDE S.A. 1.00 France Materials BECTON DICKINSON & CO COM 1.00 United States Health Care NICE SYSTEMS LTD 0.99 Israel Information Technology VARIAN MED SYS INC COM 0.99 United States Health Care SYMRISE AG 0.98 Denmark Materials RECKITT BENCK GRP 0.98 United Kingdom Consumer Staples SUNTORY BEVERAGE & FOOD LTD 0.97 Japan Consumer Staples NOVO NORDISK A/S-B 0.94 Denmark Health Care SCHNEIDER ELECTRIC S.A. -

Sony Corporation
SONY CORPORATION Notice of the Ordinary General Meeting of Shareholders to be held on June 18, 2019 To the Registered Holders of American Depositary Receipts representing shares of Common Stock of Sony Corporation (the “Corporation”): The undersigned Depositary has received a notice that the Corporation has called an ordinary general meeting of shareholders to be held in Tokyo, Japan on June 18, 2019 (the “Meeting”) for the following purposes: MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2019 (from April 1, 2018 to March 31, 2019) pursuant to the Companies Act of Japan. PROPOSALS TO BE ACTED UPON: 1. To elect 13 Directors. 2. To issue Stock Acquisition Rights for the purpose of granting stock options. EXPLANATION REGARDING THE SUBJECT MATTER OF THE MEETING MATTERS TO BE REPORTED: To receive reports on the business report, non-consolidated financial statements, consolidated financial statements, as well as audit reports on the consolidated financial statements by the Independent Auditors (certified public accountants) and the Audit Committee for the fiscal year ended March 31, 2019 (from April 1, 2018 to March 31, 2019). Note: The Consolidated Financial Statements are available on the Sony Investor Relations website. This document can be accessed at https://www.sony.net/SonyInfo/IR/stock/shareholders_meeting/Meeting102/ 1 PROPOSALS TO BE ACTED UPON: 1. To elect 13 Directors. The term of office of all 12 Directors currently in office will expire at the conclusion of the Meeting. -

Notice of Convocation of the 12Th Term Annual
Notice: This is a translation of a notice in Japanese and is made solely for the convenience of foreign shareholders. In the case of any discrepancy between the translation and the Japanese original, the latter shall prevail. [Translation] Notice of Convocation of the 12 th Term Annual Shareholders Meeting Date and Time: 10:00 a.m. on Monday, June 19, 2017 (Admission commences at: 9 a.m.) Place: “International Convention Center Pamir,” Grand Prince Hotel New Takanawa Matters to be resolved: Table of Contents First Proposal: Appropriation of Retained Earnings Notice of Convocation of the 12th Term Annual Shareholders Meeting ……………………………………………… 01 Second Proposal: Election of Six (6) Directors Reference Documents for Shareholders Meeting …………………… 07 Third Proposal: Provision of Bonus to Directors (Attachment) Business Report ……………………………………………………………… 19 Consolidated Financial Statements and Financial Statements …… 66 Audit Report …………………………………………………………………… 72 Securities Code: 4503 005_9778701172906.indd 1 2017/05/25 19:03:27 The Notice of Convocation (Securities Code 4503) May 30, 2017 To: Shareholders Notice of Convocation of the 12th Term Annual Shareholders Meeting Dear Madam/Sir: You are hereby notified that the 12th Term Annual Shareholders Meeting of Astellas Pharma Inc. (the “Company”) will be held as stated below. You are cordially invited to attend the meeting. In the event that you are unable to attend the aforesaid meeting, you may exercise your voting rights either by mail or by electronic or magnetic means (via Internet, etc.). In that case, the Company cordially requests that you consider the Reference Documents for Shareholders Meeting attached below and exercise the voting rights before 5:00 p.m. -

Press Release
Press Release Astellas Reports Full Year FY2016 Financial Results - Sales decreased (-4.4%) on a reported basis and increased approximately 2% excluding the factors associated with the transfer of the global dermatology business implemented in April 2016 and the impact of the foreign exchange; Increased core operating profit (+2.7%) and core profit (+7.3%) - Sales of XTANDI® (enzalutamide) and overall overactive bladder (“OAB”) treatments steadily increased on a constant currency basis - Sales in the Americas, EMEA1, Asia and Oceania decreased while increased on a constant currency basis. Sales in Japan decreased due to impacting factors such as a National Health Insurance (“NHI”) drug price revision - Astellas continues to create solid and resilient growth over the mid-to-long term Astellas completed the acquisition of Ganymed Pharmaceuticals AG (“Ganymed”) in December 2016 Astellas entered into a definitive agreement under which Astellas has agreed to acquire Ogeda SA (“Ogeda”) in March 2017; The procedures required for this acquisition are still in progress (as of April 2017). Tokyo, April 27, 2017 - Astellas Pharma Inc. (TSE: 4503, President and CEO: Yoshihiko Hatanaka, “Astellas”) today announced the financial results for fiscal year 2016 (“FY2016”) ended March 31, 2017. “We achieved multiple milestones including the acquisition of Ganymed last December and an agreement with Ogeda shareholders to acquire Ogeda last month. These acquisitions and other alliances will expand Astellas’ late-stage pipeline and contribute to its mid-to-long