NGFN-2) Evgenia Makrantonaki, Christos C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathologic Patterns of the Sebaceous Gland* John S
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector PATHOLOGIC PATTERNS OF THE SEBACEOUS GLAND* JOHN S. STRAUSS, M.D.t AND ALBERT M. KLIGMAN, M.D. By studying the way in which a structurecells which subsequently rupture in the fundus reacts to an imposed experimental stress, oneof the gland. Fragments of the thin eosinophilie can often better understand the changes ex-cell wall, which morphologically resemble kera- hibited in spontaneous dlsease. Much has beentin, persist in the sebum; occasionally the entire learned about the potentialities of the eccrinecell walls survive as ghosts. Furthermore, the in- and apocrine sweat units in this fashion (1—12).dividual oil droplets are separated by keratin- Previous study of the response to injury in thelike trabeculae. A dual potentiality is exhibited sebaceous gland has been restricted mainly to theby sebaceous cells in their capacity to produce changes that occur in the sebaceous duct per sefat predominantly and keratin to a minor de- (13). In this study we have followed the reac-gree. Epidermal cells have this bipotentiality tion of the sebaceous gland itself to a variety ofwith keratin as the major product. cutaneous insults and have correlated the findings with those which occur in disease states. MA.TERIALS AND METRODS The scalp and cheek of post-puberal individuals MOBPOLOGY AND FUNCTION OF were selected for study because the glands here TRN SEACEOUS GLAND are among the largest and most numerous. Biopsy 1.Morphology: The glands of the glabrous skinspecimens were obtained before the experimental are not free but are associated with hair follicles,stresses as well as at varying intervals afterwards. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

CHAPTER 4 the Integumentary System
CHAPTER 4 The Integumentary System LEARNING OBJECTIVES Upon completion of this chapter, you should be able to: • Name the two layers of the skin. • Name the accessory structures of the integumentary system. • Build and pronounce medical terms of the integumentary system. • Name the disorders and treatments relating to the integumentary system. • Name the major classifi cations of pharmacologic agents used to treat skin disorders. • Analyze and defi ne the new terms introduced in this chapter. • Interpret abbreviations associated with the integumentary system. 53 54 PART TWO • BODY SYSTEMS Introduction The largest organ of the body is the skin. The skin covers the entire body—more than 20 square feet on average—and weighs about 24 pounds. It is part of the integumentary system, which also includes the accessory structures: hair, nails, and sebaceous (oil) and sudoriferous (sweat) glands. Integumentum is Latin for “covering” or “shelter.” The physician who specializes in the diag- nosis and treatment of skin disorders is called a dermatologist (dermat/o being one of the com- bining forms for skin). Coupling the root dermat/o with the previously learned suffi x -logy gives us the term dermatology , which is the term for the specialty practice that deals with the skin. Word Elements The major word elements that relate to the integumentary system consist of various anatomical components, accessory structures, colors of the skin, and abnormal conditions. The Word Ele- ments table lists many of the roots, their meanings, and examples associated -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization -

View Sample Pages Here
3 Introduction When properly developed, communicated and implemented, guidelines improve the quality pathophysiological scars further com- of care that is provided and patient outcomes. pounded by traumatic emotional sequelae Guidelines are intended to support the health- and other comorbidities. care professional’s critical thinking skills and judgment in each case. Traumatic scars can occur as a result of acci- In addition to guiding safer, more effective and dents, acts of violence and other catastrophic consistent outcomes, the authors can only dare events (e.g. disease, burn accident and surgery). to dream that these guidelines will also support better constructed MT research methodology Over the last several decades, advancements in and designs, ultimately leading to the inclusion medical technology have led to improved surgi- of MT professionals in mainstream interprofes- cal techniques and emergency care (Blakeney & sional healthcare. Creson 2002). Simply put, more people are sur- viving injuries that would have been fatal 20 or 30 What Defines Traumatic Scarring? years ago, and an increase in survival rate means an increased need for professionals skilled in The American Psychological Association defines treating people with scars. Working successfully trauma as an emotional response to a terrible with traumatic scars requires expert navigation, event like an accident, sexual assault or natural not only of the physicality of the scar material but disaster (APA 2015). also inclusive of the whole clinical presentation. Scar or cicatrix, derived from the Greek eschara – This book will provide the information needed to meaning scab – is the fibrous replacement tis- help guide the development of that expertise. sue that is laid down following injury or disease Given the complexity of traumatic scars, where (Farlex 2012). -

Physiology of Sweat Gland Function: the Roles of Sweating and Sweat Composition in Human Health Lindsay B
COMPREHENSIVE REVIEW Physiology of sweat gland function: The roles of sweating and sweat composition in human health Lindsay B. Baker Gatorade Sports Science Institute, PepsiCo R&D Physiology and Life Sciences, Barrington, IL, USA ABSTRACT ARTICLE HISTORY The purpose of this comprehensive review is to: 1) review the physiology of sweat gland function Received 30 April 2019 and mechanisms determining the amount and composition of sweat excreted onto the skin Revised 6 June 2019 surface; 2) provide an overview of the well-established thermoregulatory functions and adaptive Accepted 8 June 2019 responses of the sweat gland; and 3) discuss the state of evidence for potential non-thermo- KEYWORDS regulatory roles of sweat in the maintenance and/or perturbation of human health. The role of Chloride; potassium; sauna; sweating to eliminate waste products and toxicants seems to be minor compared with other sodium; sweat biomarkers; avenues of excretion via the kidneys and gastrointestinal tract; as eccrine glands do not adapt to thermoregulation increase excretion rates either via concentrating sweat or increasing overall sweating rate. Studies suggesting a larger role of sweat glands in clearing waste products or toxicants from the body may be an artifact of methodological issues rather than evidence for selective transport. Furthermore, unlike the renal system, it seems that sweat glands do not conserve water loss or concentrate sweat fluid through vasopressin-mediated water reabsorption. Individuals with high NaCl concentrations in sweat (e.g. cystic fibrosis) have an increased risk of NaCl imbalances during prolonged periods of heavy sweating; however, sweat-induced deficiencies appear to be of minimal risk for trace minerals and vitamins. -

Histology and Cytochemistry of Human Skin. Xiv. the Blood Supply of the Cutaneous Glands* Richard A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector HISTOLOGY AND CYTOCHEMISTRY OF HUMAN SKIN. XIV. THE BLOOD SUPPLY OF THE CUTANEOUS GLANDS* RICHARD A. ELLIS, PH.D., WILLIAM MONTAGNA, Pn.D. AND HERBERT FANGER, M.D. Although the general blood supply to the skinout clearly against a nearly colorless background. has been mapped out in some detail (1), the exactIn thick frozen sections the entire capillary plexus vascular patterns of the human cutaneous ap-surrounding the cutaneous glands can frequently pendages need clarification. Other authors havebe seen as they emerge from their parent arteriole used injection methods, silver impregnation, and(Figs. 3, 6, 8). The endothelium of the arterioles benzidine to demonstrate the blood vessels of thehas practically no alkaline phosphatase activity, skin. None of these methods is completely satis-but enzyme activity becomes increasingly strong factory or practical in all cases. We have found,near the emergence of the capillaries and is in- however, that the capillaries supplying the skintense in the final capillary loops (9). Although it and the cutaneous appendages can be easilyis difficult to positively identify the arterioles and visualized in frozen sections with the azo-dyevenules in these preparations, recent observations technic for alkaline phosphatase (Fig. 1). Thisin our laboratory on the localization of phos- method is superior to the others used. It is simple,phorylase activity in human skin make the identi- quasi-specific, and demonstrates clearly even col-fication of arterioles easy, since the smooth mus- lapsed or blocked capillaries. Using this techniccle cells around them are rich in this enzyme (Fig. -

Skin Health and Stump Hygiene1
Skin Health and Stump Hygiene1 GILBERT H. BARNES, M.D.2 Literally the word "hygiene" connotes a loss of its protective properties. Among indi state or condition of health. But adequate hy viduals in certain occupations, we frequently giene, or good health, of the human skin pre see both manifestations of such skin reaction. sents a complex problem involving much more Housewives, mechanics, laboratory workers, than a casual acquaintance with soap and and others whose work exposes certain areas water, the concept which usually comes to of the body, particularly the hands and arms, mind when hygiene is mentioned. The func to prolonged soaking in solutions and solvents, tional state of our human integument is pretty or even in plain water, are prone to recurrent much taken for granted by most of us. We skin irritation and breakdown. In such cases, know that this two-square-yard covering will, the chemical and physiological properties of in most cases, repair itself in event of local the skin are altered to such a degree that the injury, provided infection is avoided. Cheer skin's built-in protective functions are no fully we dissolve it in strong chemical solu longer effective. Even in the absence of pro tions. We broil it in the summer sun until it longed soaking, the skin may be injured locally peels off like old birch bark. We allow it to be by contact with an irritant, such as a strong rubbed and blistered in tight shoes for vanity's acid, or with a sensitizing agent, such as sake. -
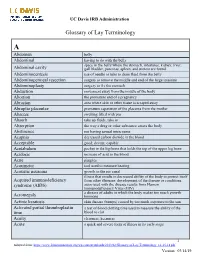
UC Davis IRB Administration
UC Davis IRB Administration Glossary of Lay Terminology A Abdomen belly Abdominal having to do with the belly space in the belly where the stomach, intestines, kidney, liver, Abdominal cavity gall bladder, pancreas, spleen, and ureters are found Abdominocentesis use of needle or tube to drain fluid from the belly Abdominoperineal resection surgery to remove the middle and end of the large intestine Abdominoplasty surgery to fix the stomach Abduction movement away from the middle of the body Abortion the premature end of a pregnancy Abrasion area where skin or other tissue is scraped away Abruptio placentae premature separation of the placenta from the mother Abscess swelling filled with pus Absorb take up fluids, take in Absorption the way a drug or other substance enters the body Abstinence not having sexual intercourse Acapnia decreased carbon dioxide in the blood Acceptable good; decent; capable Acetabulum pocket in the hip bone that holds the top of the upper leg bone Acidosis increase of acid in the blood Acne pimples Acoumeter tool used to measure hearing Acoustic neuroma growth in the ear canal illness that results in decreased ability of the body to protect itself Acquired immunodeficiency from other illnesses; development of the disease or conditions syndrome (AIDS) associated with the disease results from Human Immunodeficiency Virus (HIV) a disease of adults in which the body makes too much growth Acromegaly hormone Actinic keratosis skin disease (bumps) caused by too much exposure to the sun Activated partial thromboplastin -

Nomina Histologica Veterinaria
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Regeneration of Hair Follicles and Sebaceous Glands from the Epithelium of Scars in the Rabbit*
Regeneration of Hair Follicles and Sebaceous Glands from the Epithelium of Scars in the Rabbit* CHARLESBREEDIS (Department of Pathology, University of Pennsylvania School of Medicine, Philadelphia, Pa.) It has been generally accepted that, when all the boiling, during which time the skin was painted with iodine and hair follicles and sebaceous glands in a region of then washed with ether. A disc of skin about 25 mm. in diame ter was cut with a scissors from within the circle of ink and con adult skin are destroyed, or the entire region is centric with it. The disc included the total thickness of skin as extirpated, the new epithelium growing in from the well as the underlying panniculus carnosus. side will not regenerate these structures (14). The wire ring was now pushed through the wound into the On the basis of this concept, attempts were subcutaneous tissue and was so placed that its upward pro jecting prongs coincided with the marks on the India ink made to obtain a purely squamous sheet of epi circle. The skin was punctured at each mark with a No. 11 thelium in the rabbit for the purpose of inoculating Bard-Parker blade, thus allowing the prongs to project through it with the Shope papilloma virus. A region of skin the skin. The appearance of the anchor ring in relation to a was surgically removed from the back, and the healed wound is shown in Figure 3. The wound was protected by a glass-covered chamber wound was allowed to heal under conditions that fastened to the ring, as shown in Figure 2.