Ullmann's Fibers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing
machines Article Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing Flaviana Calignano 1,* , Massimo Lorusso 2 , Ignanio Roppolo 3 and Paolo Minetola 1 1 Department of Management and Production Engineering, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Istituto Italiano di Tecnologia, Center for Sustainable Future Technologies IIT@Polito, Corso Trento 21, 10129 Turin, Italy; [email protected] 3 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: fl[email protected]; Tel.: +39-011-090-7218 Received: 19 July 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Additive manufacturing (i.e., 3D printing) has rapidly developed in recent years. In the recent past, many researchers have highlighted the development of in-house filaments for fused filament fabrication (FFF), which can extend the corresponding field of application. Due to the limited mechanical properties and deficient functionality of printed polymer parts, there is a need to develop printable polymer composites that exhibit high performance. This study analyses the actual mechanical characteristics of parts fabricated with a low-cost printer from a carbon fibre-reinforced nylon filament. The results show that the obtained values differ considerably from the values presented in the datasheets of various filament suppliers. Moreover, the hardness and tensile strength are influenced by the building direction, the infill percentage, and the thermal stresses, whereas the resilience is affected only by the building direction. Furthermore, the relationship between the mechanical properties and the filling factor is not linear. -

The Pennsylvania State University the Graduate School AN
The Pennsylvania State University The Graduate School AN EXAMINATION OF ANALYTICAL METHODS TOWARDS THE COMPLETE ANALYSIS OF CONTAMINANTS OF EMERGING CONCERN IN WASTEWATER AND WASTEWATER IMPACTED SURFACE WATER, SOILS, AND CROPS. A Dissertation in Chemistry by Kyra A. Murrell © 2020 Kyra A. Murrell Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2020 The dissertation of Kyra A. Murrell was reviewed and approved by the following: Frank L. Dorman Associate Professor of Biochemistry and Molecular Biology Dissertation Co-Advisor Co-Chair of Committee Miriam Freedman Associate Professor of Chemistry, Meteorology and Atmospheric Science Dissertation Co-Adviser Co-Chair of Committee Paul Cremer J. Lloyd Huck Professor of Chemistry and of Biochemistry and Molecular Biology Christine Keating Distinguished Professor of Chemistry Jack Watson Professor of Soil Science, Soil Physics, Biogeochemistry Philip Bevilacqua Distinguished Professor of Chemistry and Biochemistry and Molecular Biology Department Head, Chemistry iii ABSTRACT The presence of contaminants of emerging concern (CECs) in the environment is a growing field of research for analytical environmental scientists. CECs are a class of anthropogenic pollutants not regulated by governmental agencies, and their potential deleterious environmental and human impacts are largely unknown. One of the main sources of CEC entry into the aquatic environment is wastewater treatment plant (WWTP) effluent as the treated water is often released into bodies of water, such as river and streams. Because most WWTPs were not designed to remove organic micropollutants, many CECs are poorly removed in traditional WWTPs and persist in the treated effluent waters. As a model system for study, the University Park WWTP treats the wastewater from the Penn State main campus. -

Natural Fibers and Fiber-Based Materials in Biorefineries
Natural Fibers and Fiber-based Materials in Biorefineries Status Report 2018 This report was issued on behalf of IEA Bioenergy Task 42. It provides an overview of various fiber sources, their properties and their relevance in biorefineries. Their status in the scientific literature and market aspects are discussed. The report provides information for a broader audience about opportunities to sustainably add value to biorefineries by considerin g fiber applications as possible alternatives to other usage paths. IEA Bioenergy Task 42: December 2018 Natural Fibers and Fiber-based Materials in Biorefineries Status Report 2018 Report prepared by Julia Wenger, Tobias Stern, Josef-Peter Schöggl (University of Graz), René van Ree (Wageningen Food and Bio-based Research), Ugo De Corato, Isabella De Bari (ENEA), Geoff Bell (Microbiogen Australia Pty Ltd.), Heinz Stichnothe (Thünen Institute) With input from Jan van Dam, Martien van den Oever (Wageningen Food and Bio-based Research), Julia Graf (University of Graz), Henning Jørgensen (University of Copenhagen), Karin Fackler (Lenzing AG), Nicoletta Ravasio (CNR-ISTM), Michael Mandl (tbw research GesmbH), Borislava Kostova (formerly: U.S. Department of Energy) and many NTLs of IEA Bioenergy Task 42 in various discussions Disclaimer Whilst the information in this publication is derived from reliable sources, and reasonable care has been taken in its compilation, IEA Bioenergy, its Task42 Biorefinery and the authors of the publication cannot make any representation of warranty, expressed or implied, regarding the verity, accuracy, adequacy, or completeness of the information contained herein. IEA Bioenergy, its Task42 Biorefinery and the authors do not accept any liability towards the readers and users of the publication for any inaccuracy, error, or omission, regardless of the cause, or any damages resulting therefrom. -

PEP Review 2015-06 Polyamide (Nylon) 6 and 66 Process Summary
IHS CHEMICAL PEP Review 2015-06 Polyamide (Nylon) 6 and 66 Process Summary July 2015 ihs.com PEP Review Process Economics Program Dipti Dave Senior Analyst II IHS CHEMICAL | Process Economics Program Review 2015-06 PEP Review 2015-06 Polyamide (Nylon) 6 and 66 Process Summary Dipti Dave, Senior Analyst II Abstra ct Polyamide 6 and 66 (or Nylon 6 and 66) are the most common types of polyamide available commercially. The total volume for the Nylon 6 and 66 polymerization market is 7.2 million tons in 2014, up from 6.4 million tons in 2010. Nylon 6 and 66 polymerization produces either chips or resin in uniform pellets. The chips or resin are further processed into two major applications: fibers or engineering thermoplastics (ETP). The fibers may also be directly produced from the molten state of the polymer, bypassing chip/resin production. The majority of the Nylon chip or resin production accounts for 92% of total polymerization, while fiber production (directly from melting) accounts for 8% market share. Demand is expected to grow at an average annual growth rate (AAGR) of 2.4% for Nylon 6 ETP and fiber. The AAGR for Nylon 66 ETP and fiber demand is 2.6%. Capacity additions have been taking place mostly in China. The Nylon processes have been reviewed by IHS Chemical Process Economics Program (PEP) since its inception in 1962. In this process summary, we review the key features for Nylon 6 and 66 production processes, and discuss recent technology developments and update the process economics for the following Nylon 6 and 66 stand-alone and integrated processes presented: 1. -

Synthesis and Characterization of In-Situ Nylon-6/Epoxy Blends
Synthesis and Characterization of in-situ Nylon-6/Epoxy Blends A thesis submitted to the Division of Research and Advanced Studies University of Cincinnati In partial fulfillment of the requirements for the degree of Master of Science 2016 In the Materials Science and Engineering Program, The Department of Mechanical and Materials Engineering By Anushree Deshpande B.E Polymer, University of Pune, 2011 Committee Members: Dr. Jude O. Iroh (Chair) Dr. Relva C. Buchanan Dr. Raj M. Manglik 1 ABSTRACT Epoxy is a thermosetting polymer known for its excellent adhesion, thermal stability, chemical resistance and mechanical properties. However, one of the major drawbacks of epoxies is its inherent brittleness. In order to overcome this drawback, incorporation of a thermoplastic as a second phase has proven to improve the impact strength without affecting the mechanical properties of epoxy. Researchers in the past have studied polyamide/epoxy blends in terms of blend compatibility, thermo-mechanical properties and morphology via solution blending. The current research effort employs in-situ polymerization to synthesize polyamide/epoxy blends. Blends of various compositions were synthesized by introducing Ɛ-Caprolactam (monomer of nylon-6) in the prepolymer of epoxy. All blend fractions were cured by exposing them to the same time and temperature conditions; and characterized using Dynamic Mechanical Analysis (DMA), Fourier Transform Infrared Spectroscopy (FTIR), Brookfield Viscometry, Scanning Electron Microscopy (SEM) and Thermogravimetric Analysis (TGA). DMA results show an overall increase in glass transition temperature and storage modulus in the rubbery region. FTIR results reveal maximum epoxy curing up to 15 wt% monomer loading, beyond which the plateauing of the epoxy conversion is recognized. -

Carpet Recycling
1 TheThe ParticipantParticipant willwill gaingain knowledgeknowledge inin thethe following:following: › Drivers for Carpet Recycling › General Categories of carpet recycling › Differences in Various types of recycling › Market Values of Various Recycled Products › Demand for Recycled materials from Carpet › Understanding Capital needs of Recycling › Present & Anticipated recycling capacities › Present & New Recycling technologies › Challenges & Opportunities 2 3 BroadBroad ListList ofof Drivers:Drivers: › Carpet Manufacturers › LEED building Standards –Need P. Consumer for high value/Specifications › NSF 140 › High value of P.C. content › Platinum Level highly prized: Requires Min. Post consumer content. › Platinum Level requires P.C. Carpet recycling at CARE Goal levels – Escalate every year. › Professional Specifying Commercial Community demands Sustainability › Reward most Sustainable companies with increased business: or NO business › Recycling and P. Consumer recycled content is large factor › Large National Accounts demanding sustainable initiatives: › Wal‐Mart, Home Depot, etc. › Good Old healthy competition. 4 BroadBroad ListList ofof Drivers:Drivers: › Entrepreneurs: › Willing to risk Capital for carpet recycling › They are beginning to see fairly good business model › Beginning to make money from carpet recycling › They are essential link in the value chain of processing › High Oil prices › Keeps Virgin Nylon very expensive › Cost Spread between virgin and P. Consumer is wide › Makes P. Consumer very attractive for cost savings -
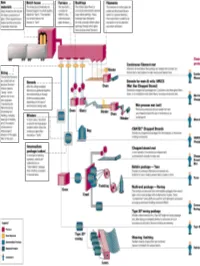
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Carbon Fiber Supply and Demand
Carbon Fiber Supply and Demand Supply of PAN based carbon fiber New players could make all the difference San Diego, California, USA October 23 2007 The ongoing shortage of PAN based carbon fibers has seen the planning, development and realization of new carbon fiber manufacturing capacity in new countries. The start up of new plant faces many hurdles, especially in the face of competition that has been in the business for many decades. However these hurdles have been overcome and this process will enable carbon fiber to become truly a commodity product, available to the vast range of current and new composite applications. Latest News (Press releases in the last 4 weeks) Supply October 11, 2007 Capacity Expansion of Carbon Fiber “TENAX®” Toho Tenax Co., Ltd. (Head Office: Bunkyo-ku, Tokyo, President: Yoshikuni Utsunomiya), which is engaged in the business of carbon fibers within the Teijin Group, has decided to add a new carbon fiber production line at Toho Tenax Europe GmbH (located at Wuppertal, Germany. October 05, 2007 SGL Group plans to triple carbon fiber capacities SGL Group plans to triple carbon fiber capacities Capacity increase up to 12,000 metric tons by 2012 Demand September 27, 2007 British Airways Green light for aircraft orders British Airways has today placed an order for 12 Airbus A380 and 24 Boeing 787 aircraft with options for a further seven Airbus A380s and18 Boeing 787s. The British Airways Order The new aircraft will replace 34 of the airline's long haul fleet and will be delivered between 2010 and 2014. The order, including options, will give the airline the ability to grow its capacity by up to four per cent per year and the flexibility to tailor its future capacity growth in line with market conditions. -

Cyclohexanone Oxime 20X29.Indd
Proprietary process technology CYCLOHEXANONE OXIME AMMOXINATION OF CYCLOHEXANONE WITH TITANIUM SILICATE (TS-1) PROPIETARY CATALYST Versalis proprietary process technologies available for licensing II 1 Our company Our commitment to excellence, in quality of our Versalis – the petrochemical subsidiary of Eni – is products and services, makes our company an active a dynamic player in its industry sector facing the partner for the growth of customers involved in multifold market needs through different skills. petrochemical business. With a history as European manufacturer with more Through engineering services, technical assistance, than 50 years of operating experience, Versalis stands marketing support and continuous innovation, our as a complete, reliable and now global supplier in the knowledge is the key strength to customize any new basic chemicals, intermediates, plastics and elastomers project throughout all phases. market with a widespread sales network. Customers can rely on this strong service-oriented Relying on continuous development in its production outlook and benefit from a product portfolio that plants as well as in its products, strengthening the strikes a perfect balance of processability and management of the knowledge gained through its long mechanical properties, performance and eco- industrial experience, Versalis has become a worldwide friendliness. licensor of its proprietary technologies and proprietary catalysts. The strong integration between R&D, Technology and Engineering departments, as well as a deep market -
Natural Fiber Composites for Space Applications
Natural fiber composites for space applications Clean Space industrial days, 25.10.2017 Julien Rion, CTO Bcomp Ltd 1700 Fribourg Switzerland ABOUT BCOMP . Founded 2011, 12 Employees . Several innovation- and start-up awards, including Swiss Economic Award 2016 . Broad customer basis in Sports & Leisure and Luxury industries (e.g. K2, Black Diamond, Nordica, Stöckli, Starboard) . Lightweighting development projects with leading Automotive OEMs . International academic/research network 07.11.2017 2 OUR VALUE PROPOSITION We deliver lightweight, high-performance materials in a renewable package . Weight reduction of up to 40% . Material cost reduction of up to 30% Flax fibres Structural part Bcomp reinforcement 07.11.2017 3 BCOMP PRODUCT PORTFOLIO bCores® ampliTex® powerRibs Services 07.11.2017 4 Why using natural fibers? . High specific stiffness . Good vibration damping properties . Probably demisable . Radio-transparent . Sustainable fibers State of the art for space applications Are natural fibers applicable for space? . Tensiles tests between -50 and 150°C . DMA tests from -150°C to 150°C . TMA tests . Outgasing tests . Moisture tests Tensile tests . High specific stiffness at low temperature . Bilinear behaviour . Loss of stiffness with temperature increase Stiffness 48 12 E1 44 11 E2 40 ET 10 36 9 32 8 28 7 24 6 20 5 16 4 [GPa] ET E1 , E2 [GPa] E1 , 12 3 8 2 4 1 0 0 -100 -50 0 50 100 150 200 T [°C] DMA tests Test on L specimens: . Constant decrease of E with T increase . Damping quite constant DMA tests 80 E-modulus: 70 . Constant decrease of E 60 with T increase 50 40 . -

Basic of Textiles
BASIC OF TEXTILES BFA(F) 202 CC 5 Directorate of Distance Education SWAMI VIVEKANAND SUBHARTI UNIVERSITY MEERUT 250005 UTTAR PRADESH SIM MOUDLE DEVELOPED BY: Reviewed by the study Material Assessment Committed Comprising: 1. Dr. N.K.Ahuja, Vice Chancellor Copyright © Publishers Grid No part of this publication which is material protected by this copyright notice may be reproduce or transmitted or utilized or store in any form or by any means now know or here in after invented, electronic, digital or mechanical. Including, photocopying, scanning, recording or by any informa- tion storage or retrieval system, without prior permission from the publisher. Information contained in this book has been published by Publishers Grid and Publishers. and has been obtained by its author from sources believed to be reliable and are correct to the best of their knowledge. However, the publisher and author shall in no event be liable for any errors, omission or damages arising out of this information and specially disclaim and implied warranties or merchantability or fitness for any particular use. Published by: Publishers Grid 4857/24, Ansari Road, Darya ganj, New Delhi-110002. Tel: 9899459633, 7982859204 E-mail: [email protected], [email protected] Printed by: A3 Digital Press Edition : 2021 CONTENTS 1. Fiber Study 5-64 2. Fiber and its Classification 65-175 3. Yarn and its Types 176-213 4. Fabric Manufacturing Techniques 214-260 5. Knitted 261-302 UNIT Fiber Study 1 NOTES FIBER STUDY STRUCTURE 1.1 Learning Objective 1.2 Introduction 1.3 Monomer, Polymer, Degree of polymerization 1.4 Student Activity 1.5 Properties of Fiber: Primary & Secondary 1.6 Summary 1.7 Glossary 1.8 Review Questions 1.1 LEARNING OBJECTIVE After studying this unit you should be able to: ● Describe the Natural Fiber.