Bibliography of Lobster Fauna of India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Download (8MB)
https://theses.gla.ac.uk/ Theses Digitisation: https://www.gla.ac.uk/myglasgow/research/enlighten/theses/digitisation/ This is a digitised version of the original print thesis. Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] ASPECTS OF THE BIOLOGY OF THE SQUAT LOBSTER, MUNIDA RUGOSA (FABRICIUS, 1775). Khadija Abdulla Yousuf Zainal, BSc. (Cairo). A thesis submitted for the degree of Doctor of Philosophy to the Faculty of Science at the University of Glasgow. August 1990 Department of Zoology, University of Glasgow, Glasgow, G12 8QQ. University Marine Biological Station, Millport, Isle of Cumbrae, Scotland KA28 OEG. ProQuest Number: 11007559 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 11007559 Published by ProQuest LLC(2018). -
Lobsters LOBSTERS§
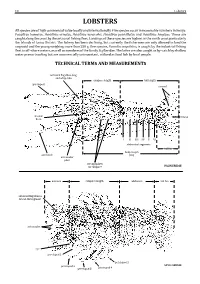
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

12 REVISED J Caveorum Profile
Document SPRFMO-III-SWG-12 Information describing Jasus caveorum fisheries relating to the South Pacific Regional Fisheries Management Organisation REVISED 20 February 2007 DRAFT 1. Overview.......................................................................................................................2 2. Taxonomy.....................................................................................................................3 2.1 Phylum..................................................................................................................3 2.2 Class.....................................................................................................................3 2.3 Order.....................................................................................................................3 2.4 Family...................................................................................................................3 2.5 Genus and species.................................................................................................3 2.6 Scientific synonyms...............................................................................................3 2.7 Common names.....................................................................................................3 2.8 Molecular (DNA or biochemical) bar coding.........................................................3 3. Species characteristics....................................................................................................3 3.1 Global distribution -

Delving Deeper Critical Challenges for 21St Century Deep-Sea Research
EUROPEAN MARINE BOARD Delving Deeper Critical challenges for 21st century deep-sea research Position Paper 22 Wandelaarkaai 7 I 8400 Ostend I Belgium Tel.: +32(0)59 34 01 63 I Fax: +32(0)59 34 01 65 E-mail: [email protected] www.marineboard.eu www.marineboard.eu European Marine Board The Marine Board provides a pan-European platform for its member organizations to develop common priorities, to advance marine research, and to bridge the gap between science and policy in order to meet future marine science challenges and opportunities. The Marine Board was established in 1995 to facilitate enhanced cooperation between European marine science organizations towards the development of a common vision on the research priorities and strategies for marine science in Europe. Members are either major national marine or oceanographic institutes, research funding agencies, or national consortia of universities with a strong marine research focus. In 2015, the Marine Board represents 36 Member Organizations from 19 countries. The Board provides the essential components for transferring knowledge for leadership in marine research in Europe. Adopting a strategic role, the Marine Board serves its member organizations by providing a forum within which marine research policy advice to national agencies and to the European Commission is developed, with the objective of promoting the establishment of the European marine Research Area. www.marineboard.eu European Marine Board Member Organizations UNIVERSITÉS MARINES Irish Marine Universities National Research Council of Italy Consortium MASTS Delving Deeper: Critical challenges for 21st century deep-sea research European Marine Board Position Paper 22 This position paper is based on the activities of the European Marine Board Working Group Deep-Sea Research (WG Deep Sea) Coordinating author and WG Chair Alex D. -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

Taxonomy, Biology and Distribution of Lobsters
Taxonomy, Biology and Distribution of Lobsters 15 Rekha Devi Chakraborty and E.V.Radhakrishnan Crustacean Fisheries Division, Central Marine Fisheries Research Institute, Kochi-682 018 Lobsters are among the most prized of fisheries resources and of significant commercial interest in many countries. Because of their high value and esteemed culinary worth, much attention has been paid to lobsters in biological, fisheries, and systematic literature. They have a great demand in the domestic market as a delicacy and is a foreign exchange earner for the country. Taxonomic status Phylum: Arthropoda Subphylum: Crustacea Class: Malacostraca Subclass: Eumalacostraca Superorder: Eucarida Order: Decapoda Suborder: Macrura Reptantia The suborder Macrura Reptantia consists of three infraorders: Astacidea (Marine lobsters and freshwater crayfishes), Palinuridea (Spiny lobsters and slipper lobsters) and Thalassinidea (mud lobsters). The infraorder Astacidea Summer School on Recent Advances in Marine Biodiversity Conservation and Management 100 Rekha Devi Chakraborty and E.V.Radhakrishnan contains three superfamilies of which only one (the Infraorder Palinuridea, Superfamily Eryonoidea, Family Nephropoidea) is considered here. The remaining two Polychelidae superfamilies (Astacoidea and parastacoidea) contain the 1b. Third pereiopod never with a true chela,in most groups freshwater crayfishes. The superfamily Nephropoidea (40 chelae also absent from first and second pereiopods species) consists almost entirely of commercial or potentially 3a Antennal flagellum reduced to a single broad and flat commercial species. segment, similar to the other antennal segments ..... Infraorder Palinuridea, Superfamily Palinuroidea, The infraorder Palinuridea also contains three superfamilies Family Scyllaridae (Eryonoidea, Glypheoidea and Palinuroidea) all of which are 3b Antennal flagellum long, multi-articulate, flexible, whip- marine. The Eryonoidea are deepwater species of insignificant like, or more rigid commercial interest. -

Approaching Marine Biodiversity Assessments Using Acoustic Methods
Listening forward: approaching marine royalsocietypublishing.org/journal/rsos biodiversity assessments using acoustic methods Review Cite this article: Mooney TA, Iorio LD, Lammers T. Aran Mooney1, Lucia Di Iorio2, Marc Lammers3, M, Lin T-H, Nedelec SL, Parsons M, Radford C, 4 5 6 Urban E, Stanley J. 2020 Listening forward: Tzu-Hao Lin , Sophie L. Nedelec , Miles Parsons , approaching marine biodiversity assessments Craig Radford7, Ed Urban8 and Jenni Stanley1 using acoustic methods. R. Soc. Open Sci. 7: 201287. 1Biology Department, Woods Hole Oceanographic Institution, 266 Woods Hole Road, http://dx.doi.org/10.1098/rsos.201287 Woods Hole, MA 02543, USA 2CHORUS Institute, Phelma Minatec, 3 parvis Louis Néel, 38000 Grenoble, France 3Hawaiian Islands Humpback Whale National Marine Sanctuary, 726 South Kihei Road, Kihei, HI 96753, USA 4Biodiversity Research Center, Academia Sinica, 128 Academia Road, Section 2, Nankang, Received: 20 July 2020 Taipei 11529, Taiwan Accepted: 5 August 2020 5Biosciences, College of Life and Environmental Sciences, Hatherly Laboratories, University of Exeter, Prince of Wales Road, Exeter EX4 4PS, UK 6Australian Institute of Marine Science, Perth, Western Australia 6009, Australia 7Institute of Marine Science, Leigh Marine Laboratory, University of Auckland, PO Box 349, Warkworth 0941, New Zealand Subject Category: 8Scientific Committee on Oceanic Research, University of Delaware, Newark, DE 19716, USA Organismal and Evolutionary Biology TAM, 0000-0002-5098-3354 Subject Areas: Ecosystems and the communities they -

Review of the Biology, Ecology and Fisheries of Palinurus Spp. Species
Cah. Biol. Mar. (2005) 46 : 127-142 Review of the biology, ecology and fisheries of Palinurus spp. species of European waters: Palinurus elephas (Fabricius, 1787) and Palinurus mauritanicus (Gruvel, 1911) Raquel GOÑI1 and Daniel LATROUITE2 (1) Centro Oceanográfico de Baleares – Instituto Español de Oceanografía. Muelle de Poniente s/n, 07080 Palma de Mallorca, Spain. Fax: 34 971 404945. E-mail: [email protected] (2) IFREMER, Centre de Brest, BP 70, 29280 Plouzané cedex, France. Fax 33 (0)2 98 22 46 53. E-mail: [email protected] Abstract: Palinurus elephas and Palinurus mauritanicus are the only species of the family Palinuridae that occur in the Northeast Atlantic and Mediterranean. Of the two, Palinurus elephas is the most abundant and accessible and has traditionally been the preferred target of lobster fisheries throughout its range. Palinurus mauritanicus has a deeper distri- bution and has been an important target of fisheries mainly in the Central Eastern Atlantic. The high unit value and the rel- ative scarcity of these species have been important obstacles to research and knowledge of their biology, ecology and fish- eries is limited. Nevertheless, over time a considerable number of studies has been conducted, though most of these are contained in university theses or in publications of limited circulation. This review is an up-to-date overview of available knowledge on the biology, ecology and fisheries of the two spiny lobster species of European waters. Résumé : Une revue sur la biologie, l’écologie et les pêcheries des espèces de Palinurus des eaux européennes : Palinurus elephas (Fabricius, 1787) et Palinurus mauritanicus (Gruvel, 1911). -

The Massive Death of Lobsters Smothered Within Their Thalassinoides Burrows: the Example of the Lower Barremian from Lusitanian Basin (Portugal)
Versão online: http://www.lneg.pt/iedt/unidades/16/paginas/26/30/209 Comunicações Geológicas (2016) 103, Especial I, 143-152 ISSN: 0873-948X; e-ISSN: 1647-581X The massive death of lobsters smothered within their Thalassinoides burrows: the example of the lower Barremian from Lusitanian Basin (Portugal) A morte em massa de lagostas sepultadas no interior das suas galerias do tipo Thalassinoides: o exemplo do Barremiano inferior da Bacia Lusitânica (Portugal) C. Neto de Carvalho 1,* Artigo original © 2014 LNEG – Laboratório Nacional de Geologia e Energia IP Original Article Abstract: Some years ago an exceptional site with the preservation of the evolutiva demonstrada pela ocorrência de Thalassinoides associados a lagostas mecochirid lobster Meyeria rapax (Harbort) within their burrows was glyphoídeos desde o Jurássico Superior ao Aptiano, e à sua progressiva described in the Early Cretaceous of Cabo Espichel sector, Lusitanian Basin, substituição pelos thalassinídeos, com algumas excepções registadas para as Portugal. The large number of articulated specimens preserved at the bottom lagostas queladas, a partir do Cretácico Superior. Esta tendência para a of Thalassinoides burrow systems in, at least, four different beds of the Boca convergência evolutiva no hábito fossorial é comparável com os padrões do Chapim Formation (lower Barremian) suggests that a sudden cyclic event evolutivos de diversificação das lagostas e dos thalassinídeos reconhecidos was responsible for the collective killing below the bottom of a confined recentemente. lagoon, and led to the very rare preservation of both burrows and producers. Palavras-chave: Meyeria rapax ; Thalassinoides suevicus ; morte colectiva; The explanation may be found in the highly heterogranular, coarse-grained convergência comportamental; Barremiano inferior; Bacia Lusitânica clastic filling of the burrow systems which represent influxes of freshwater and large volumes of siliciclastics that may had suddenly drop the salinity 1 Geopark Naturtejo da Meseta Meridional – UNESCO Global Geopark. -

104-111, March-June 1993
Indian Journal of Fisheries 40 (1,2) : 104-111, March-June 1993 Crustacean fishery resources of India - An overview C SUSEELAN' and N N PILLAI^ Central Marine Fisheries Research Institute, Kochin, Kerala 682 014 ABSTRACT The recent trend in crustacean fishery of India has been reviewed based on the landings during 1984-1992. The fishery as a \^iiole inqjroved over the years reaching a record level of 0.39 miUicn tcones in 1991. Brawn landings, which accounted for about 72% of the crustacean fishery, showed a reaunkable leap since 1988 as a result of extended fishing by shrimp trawlers over time and ^ace. and the innovative fishing in the traditional sectcr. The changed fishing pattern in the mechanized as well as aitisanal sectors, and its intact en catch and ^ecies conq)osition are outlined. The average production of 0.225 millioo tcnnes of prawns realized at present is very close to the estimated catdiable potential of the 0-50 m depth zone. Stock assessments of in:;>ortant species also reveals that the coastal >shrinq> resource of India is fiilly e;q>loited at present. Some species like Metapenaeus dohsoni, Penaeus indicus and P. semisulcatus ate overfished in their respective areas of fishery. The lobster fishery, on the whole, is in a state of decline. On the north-west coast, stock assessment of the princ^al ^ecies Panulirus polyphagus has shown that to reach the MSY level trawling e£foit would have to be considerably reduced which may not be feasible as this fishing e£foit is targeted for other resources.