Methods of Reactivity Umpolung
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UMPOLUNG in REACTIONS CATALYZED by THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung En Reacciones Catalizadas Por Enzimas Dependientes De Pirofosfato De Tiamina
Ciencia, Ambiente y Clima, Vol. 2, No. 2, julio-diciembre, 2019 • ISSN (impreso): 2636-2317 • ISSN (en línea): 2636-2333 DOI: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 UMPOLUNG IN REACTIONS CATALYZED BY THIAMINE PYROPHOSPHATE DEPENDENT ENZYMES Umpolung en reacciones catalizadas por enzimas dependientes de pirofosfato de tiamina Carlos José Boluda Emily Soto Instituto Tecnológico de Santo Domingo (INTEC), Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales, Av. de Los Área de Ciencias Básicas y Ambientales Próceres 49, Santo Domingo, República Dominicana Correo-e: [email protected] *Corresponding author: Carlos J. Boluda Darah de la Cruz Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Carolina Juncá Área de Ciencias Básicas y Ambientales Instituto Tecnológico de Santo Domingo (INTEC), Correo-e: [email protected] Área de Ciencias Básicas y Ambientales Anny Peña Correo-e: [email protected] Instituto Tecnológico de Santo Domingo (INTEC), Área de Ciencias Básicas y Ambientales Correo-e: [email protected] Recibido: 25/9/2019 • Aprobado: 19/10/2019 Cómo citar: Boluda, C. J., Juncá, C., Soto, E., de la Cruz, D., & Peña, A. (2019). Umpolung in reactions catalyzed by thiamine pyrophos- phate dependent enzymes. Ciencia, Ambiente Y Clima, 2(2), 27-42. Doi: https://doi.org/10.22206/cac.2019.v2i2.pp27-42 Abstract Resumen The temporal exchange of the electrophilic/nucleophilic El intercambio temporal del carácter electrofílico/nucleofí- character of an atom by chemical manipulation is known lico de un átomo mediante manipulación química, es cono- in organic chemistry as umpolung. This inversion of polarity cido con el vocablo alemán de umpolung. -

Modern Organic Synthesis an Introduction
Modern Organic Synthesis an Introduction G. S. Zweifel M. H. Nantz W.H. Freeman and Company Chapter 1 Synthetic Design • What is an ideal or viable synthesis, and how does one approach a synthetic project? • The overriding concern in a synthesis is the yield, including the inherent concepts of simplicity (fewest steps) and selectivity (chemoselectivity, regioselectivity, diastereoselectivity, and enantioselectivity). • This chapter outlines strategies for the synthesis of target molecules based on retrosynthetic analysis. 1 1.1 Retrosynthetic Analysis Basic Concept The symbol signifies a reverse synthetic step and is called atransform. The main transforms are disconnections, or cleavage of C-C bonds, and functional group interconversions (FGI) Retrosynthetic analysis involves the disassembly of a TM into available starting materials by sequential disconnections and functional group interconversions(FGI). Synthons are fragments resulting from disconnection of carbon-carbon bonds of the TM. The actual substrates used for the forward synthesis are the synthetic equivalents (SE). Synthetic design involves two distinct steps (1) Retrosynthetic analysis (2) Subsequent translation of the analysis into a “forward direction” synthesis. Chemical bonds can be cleaved heterolytically, homolytically, or through concerted transform. 2 Donor and Acceptor Synthons Acceptor synthon Æ carbocation (electrophilic) Donor synthon Æ carbanion (nucleophilic) Table 1.1 Common Acceptor Synthon Synthetic equivalents Common Acceptor Synthon Synthetic equivalents 3 Table 1.2 Common Donor Synthons Common Donor Synthon Synthetic equivalents Retrosynthetic Analysis A Synthesis A 4 Retrosynthetic Analysis B Synthesis B Alternating Polarity Disconnections The presence of a heteroatom in a molecule imparts a pattern of electrophilicity and nucleophilicity to the atom of the molecule. The concept of alternating polarities or latent polarities (imaginary chargies) often enables one to identify the best positions to make a disconnection within a complex molecule. -

Protokoll Handledarmöte 2020-02-14
http://www.diva-portal.org Postprint This is the accepted version of a paper published in Organic Letters. This paper has been peer-reviewed but does not include the final publisher proof-corrections or journal pagination. Citation for the original published paper (version of record): Colas, K., dos Santos, A C., Mendoza, A. (2019) i-Pr2-NMgCl·LiCl Enables the Synthesis of Ketones by Direct Addition of Grignard Reagents to Carboxylate Anions Organic Letters, 21(19): 7908-7913 https://doi.org/10.1021/acs.orglett.9b02899 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:su:diva-175818 i-Pr2NMgCl·LiCl Enables the Synthesis of Ketones by Direct Addi- tion of Grignard Reagents to Carboxylate Anions Kilian Colas, A. Catarina V. D. dos Santos and Abraham Mendoza* Department of Organic Chemistry, Stockholm University, Arrhenius Laboratory, 106 91 Stockholm (Sweden) Supporting Information Placeholder direct Grignard - carboxylate coupling 1 R MgX + i-Pr2NMgCl•LiCl 1 MgX ( ) R * CO2 i-Pr i-Pr L L O [Mg] N O ideal ( ) * Mg Li (*) sources 1 1 2 R O Cl R R R2 L O ketone [proposed structure] products base R1 OH [in-situ from commercial] ◾ >30 examples ◾ scalable ◾ facile isotopic-labelling ABSTRACT: The direct preparation of ketones from carboxylate anions is greatly limited by the required use of organolithium reagents or activated acyl sources that need to be independently prepared. Herein, a specific magnesium amide additive is used to activate and control the addition of more tolerant Grignard reagents to carboxylate anions. -
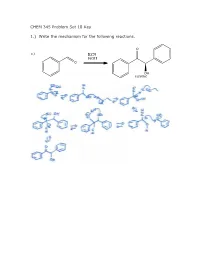
CHEM 345 Problem Set 18 Key 1.) Write the Mechanism for The
CHEM 345 Problem Set 18 Key 1.) Write the mechanism for the following reactions. O a.) KCN EtOH O OH racemic 1.) Write the mechanism for the following reactions. b.) KCN AcOH O NC OH racemic O c.) N S R O NEt3 OH racemic 2.) What is the structure of AcOH?Why does changing the solvent from EtOH to AcOH make such a big difference? O OH AcOH acetic acid The pKa of acetic acid is approximately 5. The pKa of ethanol is approximately 15. When you take a proton off of ethanol, you generate ethoxide which is about 1010 times stronger of a base than acetate. 3.) Give two instances when you need to use the thiazolium salt and triethylamine rather than KCN and EtOH. If the aldehydes contain an enolizable proton then you cannot use KCN/EtOH, instead you must use the thiazolium. Also, if the electrophile is a Michael acceptor to give a 1,4 dicarbonyl, then the thiazolium catalyst should be used. 4.) Break the following compound down as far as you can using Aldol, Michael, and Claisen reactions. Above each retrosynthetic arrow, write the name of the reaction. O HO O HO Aldol O Michael HO O O HO O Aldol O O Aldol HO O O HO Michael O O O Aldol There are other possibilities for order. O O HO Aldol O O 5.) Synthesize the following molecules. All carbons in the molecules must come from benzene or compounds with 5C’s or less. a.) O H2SO4 O HNO3 O2N AlCl3 O O SOCl2 HO Cl H2CrO4 1.) BuLi, Et2O + O 2.) H3O HO b.) O O O Cl + H3O NaOEt, EtOH O O O O Cl O AlCl3 Cl Cl AlCl3 Cl2 c.) O OMe NaOMe MeOH O O 1.) POCl3, DMF 2.) H2O OMe OMe O AlCl3 MeI Cl ONa HCl NaOH ZnHg 1.) NaOH + mcpba O 2.) H3O O OH O O AlCl3 Cl 6.) Write the mechanism for the following reactions. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

Synthesis Infographic (Revised)
Organic Synthesis A problem–solving guide for Organic Chemistry I/II ? Goal: propose a synthesis of a target molecule H OH N using given starting materials and any other N reagents you need. starting target materials molecule These key strategies should guide your approach:1 Mapping • Systematically label all the atoms in the ? 1 starting materials, then identify those atoms in H OH N 6 N the target molecule 2 4 5 7 6 1 3 5 7 2 3 4 • Use landmarks (heteroatoms, functional groups) to help you Identifying bonds formed/broken ? and atoms added/removed H OH 1 N 6 N Having labeled atoms, it becomes easier to see: 2 4 5 7 6 1 3 5 7 2 3 4 bonds formed: C7–N2 σ, C6–O σ atoms added • where bonds have been formed/broken bonds broken: C6–C7 π atoms removed • where atoms (including protons) have been added/removed ? Regiochemical and stereochemical analysis H OH 1 N 6 N 2 4 5 7 6 • Look for regiochemical patterns that can 1 3 5 7 2 3 4 provide ideas for synthetic strategies target molecule is 1,2–difunctionalized at C6–C7 • Look for changes in configuration at C7–N2 bond formed at less substituted C of propylene stereocenters (not always applicable) reaction: base–catalyzed epoxide opening 1 OH OH 1 Synthon-based retrosynthetic analysis N + N 6 2 4 6 2 4 Construct hypothetical starting materials for 5 7 3 5 7 3 • synthons forming required bonds 1 This process is called constructing synthons, O δ- • + + HN 6 6 δ 2 4 and it is the basis of retrosynthetic analysis 5 7 5 7 3 possible reagents • Synthons are a tool for choosing reagents 1Based in part on strategies observed in successful students’ responses to synthetic Flynn Research Group 2017 – FlynnResearchGroup.com problems on exams; see: Bodé, N. -

Bsc Chemistry
__________________________________________________________________________________________________ Subject Chemistry Paper No and Title 14: Organic Chemistry –IV (Advance Organic Synthesis and Supramolecular Chemistry and carbocyclic rings) Module No and 2: Synthons, Synthetic equivalents and Title Retrosynthetic analysis Module Tag CHE_P14_M2 CHEMISTRY Paper No. 14: Organic Chemistry –IV (Advance Organic Synthesis and Supramolecular Chemistry and carbocyclic rings) Module No. 2: Synthons, Synthetic equivalents and Retrosynthetic analysis __________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Synthons and Synthetic equivalents 4. Retrosynthetic analysis 5. Summary CHEMISTRY Paper No. 14: Organic Chemistry –IV (Advance Organic Synthesis and Supramolecular Chemistry and carbocyclic rings) Module No. 2: Synthons, Synthetic equivalents and Retrosynthetic analysis __________________________________________________________________________________________________ 1. Learning Outcomes After studying this module, you shall be able to: Know about synthons Know about synthetic equivalents Know about retrosynthetic analysis. Study the steps involved in retrosynthetic analysis. Know the routine for designing the synthesis. 2. Introduction Organic chemistry is a branch of chemistry which deals with the study of carbon and its compounds. The study includes the understanding of synthesis, structure, properties, and reactions of organic compounds. -

CHEM 4000 Topic 2: Functional Group Oriented Bond-Sets
CHEMISTRY 4000 Topic #2: Functional Group Oriented Bond-Sets Spring 2019 Dr. Susan Findlay Synthons for Polar Bond Formation Once we identify a bond-set (set of retrosynthetic disconnections), we have to be able to generate a corresponding set of forward reactions. (So much so that knowledge of an available set of forward reactions will have heavily influenced choice of bond-set.) Usually, the forward reaction is a polar bond formation so it involves a nucleophile and an electrophile. First, identify which of the two pieces will serve as nucleophile and which will serve as electrophile. X – X C – + H C or + 2 ? In this example, one option should appear substantially better than the other. In some cases, both options are feasible – but you still have to choose one to try first! 2 Synthons for Polar Bond Formation The functional groups in the target will tend to dictate which piece serves as the nucleophile and which serves as the electrophile (hence the term ‘functional group oriented bond-set’). Each piece is referred to as a synthon. The nucleophilic piece is the donor synthon (or d-synthon). The electrophilic piece is the acceptor synthon (or a-synthon). In the example on the previous page, the aromatic ring dictated the choice of donor and acceptor synthons. What if, instead of disconnecting next to the aromatic ring, we had chosen to disconnect at the next C-C bond in the chain? 3 Synthons for Polar Bond Formation Consider the influence of a carbonyl group on a nearby retrosynthetic disconnection. There are three reasonable choices for disconnections in the vicinity of a carbonyl: O O X – + H C 2 O O – C H 2 + X O O – + H C 2 4 See the end of this set of notes for more on reactions corresponding to the third disconnection. -

Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— a Literature Review
Molecules 2013, 18, 11873-11903; doi:10.3390/molecules181011873 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Nuclear Magnetic Resonance Approaches in the Study of 2-Oxo Acid Dehydrogenase Multienzyme Complexes— A Literature Review Sowmini Kumaran 1, Mulchand S. Patel 2 and Frank Jordan 1,* 1 Department of Chemistry, Rutgers University, Newark, NJ 07102, USA 2 Department of Biochemistry, School of Medicine and Biomedical Sciences, State University of New York at Buffalo, Buffalo, NY 14214, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-973-353-5470; Fax: +1-973-353-1264. Received: 30 July 2013; in revised form: 14 September 2013 / Accepted: 16 September 2013 / Published: 26 September 2013 Abstract: The 2-oxoacid dehydrogenase complexes (ODHc) consist of multiple copies of three enzyme components: E1, a 2-oxoacid decarboxylase; E2, dihydrolipoyl acyl-transferase; and E3, dihydrolipoyl dehydrogenase, that together catalyze the oxidative decarboxylation of 2-oxoacids, in the presence of thiamin diphosphate (ThDP), coenzyme A 2+ + (CoA), Mg and NAD , to generate CO2, NADH and the corresponding acyl-CoA. The structural scaffold of the complex is provided by E2, with E1 and E3 bound around the periphery. The three principal members of the family are pyruvate dehydrogenase (PDHc), 2-oxoglutarate dehydrogenase (OGDHc) and branched-chain 2-oxo acid dehydrogenase (BCKDHc). In this review, we report application of NMR-based approaches to both mechanistic and structural issues concerning these complexes. These studies revealed the nature and reactivity of transient intermediates on the enzymatic pathway and provided site-specific information on the architecture and binding specificity of the domain interfaces using solubilized truncated domain constructs of the multi-domain E2 component in its interactions with the E1 and E3 components. -

2614-2625 Research Article Synthon Disconnection Strategy for Th
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2012, 4(5):2614-2625 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 Synthon Disconnection Strategy for the Synthesis Design of “Coelenterazine”- A Bioluminescent Marine Natural Product used in Bioassays Chittaranjan Bhanja 1*, Subhendu Chakroborty 2 1Department of Chemistry, Utkal University, Bhubaneswar-751004, Odisha, India 2Department of Chemistry, Ravenshaw University, Cuttack-753003, Odisha, India _________________________________________________________________________________ ABSTRACT Synthon disconnection strategy is a problem solving technique in the planning of organic syntheses. This strategy, developed by Prof. E.J.Corey of Harvard University, plays vital role in the synthesis design of many bioactive natural products used in analytical biochemistry, molecular biology and drug discovery process. Keeping an overview on the published works in both journals and patent literatures, a good number of synthesis schemes have been proposed based on this strategy for a bioluminescent natural product ‘Coelenterazine’ isolated from marine organism jellyfish Aequorea victoria that finds extensive applications in bioassays. The proposed synthesis planning being a theoretical investigation, the actual laboratory execution requires the cross examination of a considerable number of factors such as reactions, reagents and order of events. In actual practice, generally that route is most feasible which is cost-effective, safe, and easy to carry out and gives maximum yield in a short reaction time. Keywords: Anti-oxidant, Bioassays, Bioluminescence, Coelenterazine, Synthon disconnection strategy, Retrosynthetic analysis. _________________________________________________________________________________ INTRODUCTION Organic synthesis is one of the major activities of organic chemists and the planning of synthesis is an intellectual task where chemist’s imagination, art, creativity and knowledge need to be explored. -

Assessing the Thiamine Diphosphate Dependent Pyruvate Dehydrogenase E1 Subunit for Carboligation Reactions with Aliphatic Ketoacids
International Journal of Molecular Sciences Article Assessing the Thiamine Diphosphate Dependent Pyruvate Dehydrogenase E1 Subunit for Carboligation Reactions with Aliphatic Ketoacids Stefan R. Marsden, Duncan G. G. McMillan and Ulf Hanefeld * Biokatalyse, Afdeling Biotechnologie, Technische Universiteit Delft, Van der Maasweg 9, 2629HZ Delft, The Netherlands; [email protected] (S.R.M.); [email protected] (D.G.G.M.) * Correspondence: [email protected] Received: 12 October 2020; Accepted: 12 November 2020; Published: 16 November 2020 Abstract: The synthetic properties of the Thiamine diphosphate (ThDP)-dependent pyruvate dehydrogenase E1 subunit from Escherichia coli (EcPDH E1) was assessed for carboligation reactions with aliphatic ketoacids. Due to its role in metabolism, EcPDH E1 was previously characterised with respect to its biochemical properties, but it was never applied for synthetic purposes. Here, we show that EcPDH E1 is a promising biocatalyst for the production of chiral α-hydroxyketones. WT EcPDH E1 shows a 180–250-fold higher catalytic efficiency towards 2-oxobutyrate or pyruvate, respectively, in comparison to engineered transketolase variants from Geobacillus stearothermophilus (TKGST). Its broad active site cleft allows for the efficient conversion of both (R)- and (S)-configured α-hydroxyaldehydes, next to linear and branched aliphatic aldehydes as acceptor substrates under kinetically controlled conditions. The alternate, thermodynamically controlled self-reaction of aliphatic aldehydes was shown to be limited to low levels of conversion, which we propose to be due to their large hydration constants. Additionally, the thermodynamically controlled approach was demonstrated to suffer from a loss of stereoselectivity, which makes it unfeasible for aliphatic substrates. Keywords: Thiamine diphosphate; transketolase; C-C bond formation; kinetic control; acyloins 1. -

Synthi: a New Open-Source Tool for Synthon-Based Library Design
Preprint___________________________ SynthI: a new open-source tool for synthon-based library design Yuliana Zabolotna1, Dmitriy M.Volochnyuk3,6, Sergey V.Ryabukhin4,6, Kostiantyn Gavrylenko5,6, Dragos Horvath1, Klimchuk Olga1, Olexandre Oksiuta3,7, Gilles Marcou1, Alexandre Varnek1,2 * ____________________________________________________________________________________________ Abstract: Most of the existing computational tools for library design are focused on the generation, rational selection, and combination of promising structural motifs to form members of the new library. However, the absence of a direct link between the chemical space of the retrosynthetically generated fragments and the pool of available reagents makes such approaches appear as rather theoretical and reality-disconnected. In this context, here we present a new open-source toolkit for library design, called Synthons Interpreter or SynthI that allows merging those two chemical spaces into a single synthons space. Here synthons are defined as the actual fragments with valid valences and special labels, defining the position and nature of reactive centers. They can be issued from either the “break-up” of reference compounds according to retrosynthetic rules, or real reagents/BBs, after leaving groups transformation. Such an approach not only enables the design of synthetically accessible libraries and analogs generation but also facilitates BB analysis in the medicinal chemistry context. SynthI code is available in https://github.com/Laboratoire-de-Chemoinformatique/SynthI