Appendix 15-B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Columbia Regional Guide Cat
National Marine Weather Guide British Columbia Regional Guide Cat. No. En56-240/3-2015E-PDF 978-1-100-25953-6 Terms of Usage Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Disclaimer: Her Majesty is not responsible for the accuracy or completeness of the information contained in the reproduced material. Her Majesty shall at all times be indemnified and held harmless against any and all claims whatsoever arising out of negligence or other fault in the use of the information contained in this publication or product. Photo credits Cover Left: Chris Gibbons Cover Center: Chris Gibbons Cover Right: Ed Goski Page I: Ed Goski Page II: top left - Chris Gibbons, top right - Matt MacDonald, bottom - André Besson Page VI: Chris Gibbons Page 1: Chris Gibbons Page 5: Lisa West Page 8: Matt MacDonald Page 13: André Besson Page 15: Chris Gibbons Page 42: Lisa West Page 49: Chris Gibbons Page 119: Lisa West Page 138: Matt MacDonald Page 142: Matt MacDonald Acknowledgments Without the works of Owen Lange, this chapter would not have been possible. -
Bcts Dcr, Dsc
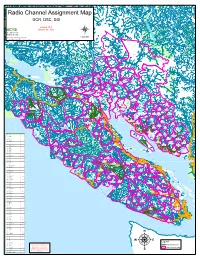
Radio Channel Assignment Map DCR, DSC, DSI Version 10.8 BCTS January 30, 2015 BC Timber Sales W a d d i Strait of Georgia n g t o n G l a 1:400,000 c Date Saved: 2/3/2015 9:55:56 AM i S e c r a r Path: F:\tsg_root\GIS_Workspace\Mike\Radio_Frequency\Radio Frequency_2015.mxd C r e e k KLATTASINE BARB HO WARD A A T H K O l MTN H O M l LANDMAR K a i r r C e t l e a n R CAMBRIDG E t e R Wh i E C V A r I W R K A 7 HIDD EN W E J I C E F I E L D Homathko r C A IE R HEAK E T STANTON PLATEAU A G w r H B T TEAQ UAHAN U O S H N A UA Q A E 8 T H B R O I M Southgate S H T N O K A P CUMSACK O H GALLEO N GUNS IGHT R A E AQ V R E I T R r R I C V E R R MT E H V a RALEIG H SAWT rb S tan I t R o l R A u e E HO USE r o B R i y l I l V B E S E i 4 s i R h t h o 17 S p r O G a c l e Bear U FA LCO N T H G A Stafford R T E R E V D I I R c R SMIT H O e PEAK F Bear a KETA B l l F A T SIR FRANCIS DRAKE C S r MT 2 ke E LILLO OE T La P L rd P fo A af St Mellersh Creek PEAKS TO LO r R GRANITE C E T ST J OHN V MTN I I V E R R 12 R TAHUMMING R E F P i A l R Bute East PORTAL E e A L D S R r A E D PEAK O O A S I F R O T T Glendale 11 R T PRATT S N O N 3 S E O M P Phillip I I T Apple River T O L A B L R T H A I I SIRE NIA E U H V 11 ke Po M L i P E La so M K n C ne C R I N re N L w r ro ek G t B I OSMINGTO N I e e Call Inlet m 28 R l o r T e n T E I I k C Orford V R E l 18 V E a l 31 Toba I R C L R Fullmore 5 HEYDON R h R o George 30 Orford River I Burnt Mtn 16 I M V 12 V MATILPI Browne E GEORGE RIVER E R Bute West R H Brem 13 ke Bute East La G 26 don ey m H r l l e U R -

3LMANUSCRIPT REPORT SERIES No. 36
DFO - L bra y MPOBibio heque II 1 111111 11 11 11 V I 1 120235441 3LMANUSCRIPT REPORT SERIES No. 36 Some If:eat/viz& 3,5,unamia, Olt the Yacific ettadt of South and ✓ cuith anwitica, T. S. Murty, S. 0. Wigen and R. Chawla Marine Sciences Directorate 975 Department of the Environment, Ottawa Marine Sciences Directorate Manuscript. Report Series No. 36 SOME FEATURES OF TSUNAMIS ON THE PACIFIC COAST OF SOUTH AND NORTH AM ERICA . 5 . Molly S . O. Wigen and R. Chawla 1975 Published by Publie par Environment Environnement Canada Canada I' Fisheries and Service des !Aches Marine Service et des sciences de la mer Office of the Editor Bureau du fiedacteur 116 Lisgar, Ottawa K1 A Of13 1 Preface This paper is to be published in Spanish in the Proceedings of the Tsunami Committee XVII Meeting, Lima, Peru 20-31 Aug. 1973, under the International Association of Seismology and Physics of the Earth Interior. 2 Table of Contents Page Abstract - Resume 5 1. Introduction 7 2. Resonance characteristics of sonic inlets on the Pacific Coast of Soulh and North America 13 3. Secondary undulations 25 4. Tsunami forerunner 33 5. Initial withdrawal of water 33 6. Conclusions 35 7. References 37 3 4 i Abstract In order to investigate the response of inlets to tsunamis, the resonance characteristics of some inlets on the coast of Chile have been deduced through simple analytical considerations. A comparison is made with the inlets of southeast Alaska, the mainland coast of British Columbia and Vancouver Island. It is shown that the general level of intensif yy of secondary undulations is highest for Vancouver Island inlets, and least for those of Chile and Alaska. -

Copyright (C) Queen's Printer, Victoria, British Columbia, Canada
B.C. Reg. 38/2016 O.C. 112/2016 Deposited February 29, 2016 effective February 29, 2016 Water Sustainability Act WATER DISTRICTS REGULATION Note: Check the Cumulative Regulation Bulletin 2015 and 2016 for any non-consolidated amendments to this regulation that may be in effect. Water districts 1 British Columbia is divided into the water districts named and described in the Schedule. Schedule Water Districts Alberni Water District That part of Vancouver Island together with adjacent islands lying southwest of a line commencing at the northwest corner of Fractional Township 42, Rupert Land District, being a point on the natural boundary of Fisherman Bay; thence in a general southeasterly direction along the southwesterly boundaries of the watersheds of Dakota Creek, Laura Creek, Stranby River, Nahwitti River, Quatse River, Keogh River, Cluxewe River and Nimpkish River to the southeasterly boundary of the watershed of Nimpkish River; thence in a general northeasterly direction along the southeasterly boundary of the watershed of Nimpkish River to the southerly boundary of the watershed of Salmon River; thence in a general easterly direction along the southerly boundary of the watershed of Salmon River to the southwesterly boundary thereof; thence in a general southeasterly direction along the southwesterly boundaries of the watersheds of Salmon River and Campbell River to the southerly boundary of the watershed of Campbell River; thence in a general easterly direction along the southerly boundaries of the watersheds of Campbell River and -

Original Field Data and Traverse Notes Must Be Provided by the Licensee
Ministry of Forests, Lands, Natural Resource Operations and Rural Development Minister’s Office MEMORANDUM Cliff: 259440 Ref: 280-20 November 25, 2020 To: Sharon Hadway, Regional Executive Director, West Coast Allan Johnsrude, Regional Executive Director, South Coast From: The Honourable Doug Donaldson Minister of Forests, Lands, Natural Resource Operations and Rural Development Re: New Coast Appraisal Manual I hereby approve the new Coast Appraisal Manual and attach a copy for your use. The manual is available at the following link: https://www2.gov.bc.ca/gov/content/industry/forestry/competitive-forest-industry/ timber-pricing/coast-timber-pricing/coast-appraisal-manual-and-amendments This manual will come into force on December 15, 2020. Further amendments or revisions to this manual require my approval. Minister pc: Melissa Sanderson, Assistant Deputy Minister, Forest Policy and Indigenous Relations Division Jim Schafthuizen, Executive Director, Forest Policy and Indigenous Relations Division Allan Bennett, Director, Timber Pricing Branch TIMBER PRICING BRANCH Coast Appraisal Manual Effective December 15, 2020 This manual is intended for the use of individuals or companies when conducting business with the British Columbia Government. Permission is granted to reproduce it for such purposes. This manual and related documentation and publications, are protected under the Federal Copyright Act. They may not be reproduced for sale or for other purposes without the express written permission of the Province of British Columbia. Coast Appraisal Manual Highlights New Coast Appraisal Manual Highlights The new Coast Appraisal Manual includes clarification to policy, an update to the market pricing system, and an update of the tenure obligation adjustments and specified operations for December 15, 2020 onward. -

2008/2009 Has Been a Pivotal Moment in Duct Business in Their Territory
CARRIER SEKANI TRIBAL COUNCIL CSTC Member Nations and Directors of the Board (as of July, 2009) 08-09Annual Report Box 36 Fort Fraser, BC V0J 1N0 Phone: (250) 690-7211 Fax: (250) 690-7316 Yin’krah Hun’zu Chief Larry Nooski Nadleh Whut’en First Nation Beautiful Earth TABLE OF CONTENTS PAGE Box 670 Fort St. James, BC V0J 1P0 Carrier Sekani Tribal Council’s Board of Directors .................1 Phone: (250) 648-3212 Box 1329 Fax: (250) 648-3250 Tribal Chief David Luggi Report .................................................... 2-5 Fort St. James, BC V0J 1P0 Phone: (250) 996-7171 Chief Thomas Alexis Vice-Tribal Chief Catherine Lessard Report ............................6 Fax: (250) 996-8010 Tl’azt’en Nation general Manager Jason Morgan’s Report .............................. 6-8 Chief Fred Sam Chairpersons for CSTC AGA 2009 .................................................8 Nak’azdli Band Elders Report • Cheryl Webster, Youth Mentoring Coordinator ...................................9-10 Fisheries Report • Bill Shepert, Program Manager ..........................................................11-12 • Christina Ciesielski, Senior Program Technician .............................. 11-12 Box 9000 RR 1, Site 12, Comp. 26 Burns Lake, BC V0J 1E0 Land Use Planning Report Vanderhoof, BC V0J 3A0 Phone: (250) 692-7717 Phone: (250) 567-9293 • Jaime Sanchez, Land Use Planning Coordinator.....................................13 Fax: (250) 692-4214 Fax: (250) 567-2998 • Janine Luggi, Conservaion Stratgy Coordinator .....................................13 -

Download The
THE CHAETOGNATHS OP WESTERN CANADIAN COASTAL WATERS by HELEN ELIZABETH LEA A THESIS SUBMITTED IN PARTIAL FULFILMENT OP THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in the Department of ZOOLOGY We accept this thesis as conforming to the standard required from candidates for the degree of MASTER OF ARTS Members of the Department of Zoology THE UNIVERSITY OF BRITISH COLUMBIA October, 1954 ABSTRACT A study of the chaetognath population in the waters of western Canada was undertaken to discover what species were pre• sent and to determine their distribution. The plankton samples examined were collected by the Institute of Oceanography of the University of British Columbia in the summers of 1953 and 1954 from eleven representative areas along the entire coastline of western Canada. It was hoped that the distribution study would correlate with fundamental oceanographic data, and that the pre• sence or absence of a given species of chaetognath might prove to be an indicator of oceanographic conditions. Four species of chaetognaths, representing two genera, were found to be pre• sent. One species, Sagitta elegans. was the most abundant and widely distributed species, occurring at least in small numbers in all the areas sampled. It was characteristic of the mixed coastal waters over the continental shelf and of the inland waters. Enkrohnla hamata. an oceanic form, occurred in most regions in small numbers as an immigrant, and was abundant to- ward the edge of the continental shelf. Sagitta lyra. strictly a deep sea species, was found only in the open waters along the outer coasts, and a few specimens of Sagitta decipiens. -

Hail the Columbia III
Hail the Columbia III Toronto , Ontario , Canada Friday, June 20, 2008 TO VANCOUVER AND BEYOND: About a year earlier, my brother Peter and his wife Lynn, reported on a one-of-a-kind cruise adventure they had in the Queen Charlotte Strait area on the inside passage waters of British Columbia. Hearing the stories and seeing the pictures with which they came back moved us to hope that a repeat voyage could be organized. And so we found ourselves this day on a WestJet 737 heading for Vancouver. A brief preamble will help set the scene: In 1966 Peter and Lynn departed the civilization of St. Clair Avenue in Toronto, for the native community of Alert Bay, on Cormorant Island in the Queen Charlotte Strait about 190 miles, as the crow flies, north-west of Vancouver. Peter was a freshly minted minister in the United Church of Canada and an airplane pilot of some experience. The United Church had both a church and a float-equipped airplane in Alert Bay. A perfect match. The purpose of the airplane was to allow the minister to fly to the something over 150 logging camps and fishing villages that are within a couple hundred miles of Alert Bay and there to do whatever it is that ministers do. This was called Mission Service. At the same time the Anglican Church, seeing no need to get any closer to God than they already were, decided to stay on the surface of the earth and so Horseshoe Bay chugged the same waters in a perky little ship. -

Flea Village—1
Context: 18th-century history, west coast of Canada Citation: Doe, N.A., Flea Village—1. Introduction, SILT 17-1, 2016. <www.nickdoe.ca/pdfs/Webp561.pdf>. Accessed 2016 Nov. 06. NOTE: Adjust the accessed date as needed. Notes: Most of this paper was completed in April 2007 with the intention of publishing it in the journal SHALE. It was however never published at that time, and further research was done in September 2007, but practically none after that. It was prepared for publication here in November 2016, with very little added to the old manuscripts. It may therefore be out-of-date in some respects. It is 1 of a series of 10 articles and is the final version, previously posted as Draft 1.5. Copyright restrictions: Copyright © 2016. Not for commercial use without permission. Date posted: November 9, 2016. Author: Nick Doe, 1787 El Verano Drive, Gabriola, BC, Canada V0R 1X6 Phone: 250-247-7858 E-mail: [email protected] Into the labyrinth…. Two expeditions, one led by Captain Vancouver and the other led by Comandante Galiano, arrived at Kinghorn Island in Desolation Sound from the south on June 25, 1792. Their mission was to survey the mainland coast for a passage to the east—a northwest passage. At this stage of their work, they had no idea what lay before them as the insularity of Vancouver Island had yet to be established by Europeans. The following day, all four vessels moved up the Lewis Channel and found a better anchorage in the Teakerne Arm. For seventeen days, small-boat expeditions set out from this safe anchorage to explore the Homfray Channel, Toba Inlet, Pryce Channel, Bute Inlet, and the narrow passages leading westward through which the sea flowed back and forth with astounding velocity. -

Oceanographic and Environmental Conditions in the Discovery Islands, British Columbia
Canadian Science Advisory Secretariat (CSAS) Research Document 2017/071 National Capital Region Oceanographic and environmental conditions in the Discovery Islands, British Columbia P.C. Chandler1, M.G.G. Foreman1, M. Ouellet2, C. Mimeault3, and J. Wade3 1Fisheries and Oceans Canada Institute of Ocean Sciences 9860 West Saanich Road Sidney, British Columbia, V8L 5T5 2Fisheries and Oceans Canada Marine Environmental Data Section, Ocean Science Branch 200 Kent Street Ottawa, Ontario, K1A 0E6 3Fisheries and Oceans Canada Aquaculture, Biotechnology and Aquatic Animal Health Science Branch 200 Kent Street Ottawa, Ontario, K1A 0E6 December 2017 Foreword This series documents the scientific basis for the evaluation of aquatic resources and ecosystems in Canada. As such, it addresses the issues of the day in the time frames required and the documents it contains are not intended as definitive statements on the subjects addressed but rather as progress reports on ongoing investigations. Research documents are produced in the official language in which they are provided to the Secretariat. Published by: Fisheries and Oceans Canada Canadian Science Advisory Secretariat 200 Kent Street Ottawa ON K1A 0E6 http://www.dfo-mpo.gc.ca/csas-sccs/ [email protected] © Her Majesty the Queen in Right of Canada, 2017 ISSN 1919-5044 Correct citation for this publication: Chandler, P.C., Foreman, M.G.G., Ouellet, M., Mimeault, C., and Wade, J. 2017. Oceanographic and environmental conditions in the Discovery Islands, British Columbia. DFO Can. Sci. Advis. -
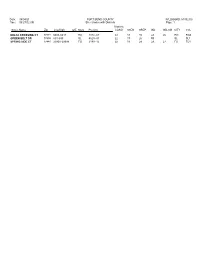
1 Street Index with Districts FORT BEND
Date: 08/04/21 FORT BEND COUNTY PA_BOOKD (v100210) Time: 09:27:02 AM Street Index with Districts Page: 1 Districts Street NameZip Low/High O/EMuni Precinct CONG SSEN SREP ISD ISD-SM CITY CCL DELTA CROSSING CT 77471 6400-6417 RO 1074 -01 22 18 85 LA L6 RO RO4 GREEN BELT DR 77498 601-699 SL 4029 -01 22 17 26 FB SL SL1 SPRING SIDE CT 77441 29900-29998 FU 3149 -15 22 18 28 LA L7 FU FU1 Date: 08/04/21 FORT BEND COUNTY PA_BOOKD (v100210) Time: 09:27:02 AM Street Index with Districts Page: 2 Districts Street NameZip Low/High O/EMuni Precinct CONG SSEN SREP ISD ISD-SM CITY CCL W 1ST 77477 10400-11104 ST 2088 -01 09 13 27 ST ST 1ST ST 77498 100-199 SL 4029 -01 22 17 26 FB SL SL1 1ST ST 77471 200-999 RO 1048 -06 22 18 85 LA L1 RO RO1 1ST ST 77471 1000-1098 E RO 1048 -07 22 18 85 LA L1 RO RO2 1ST ST 77471 1101-1499 O RO 1037 -01 22 18 85 LA L1 RO RO3 1ST ST 77471 1304-2512 E RO 1012 -03 22 18 85 LA L1 RO RO2 1ST ST 77471 1501-2299 O RO 1037 -01 22 18 85 LA L1 RO RO3 1ST ST 77477 2200-2298 E ST 2088 -01 09 13 27 ST ST 1ST ST 77477 2201-2299 O ST 2088 -01 09 13 27 ST ST 1ST ST 77477 2300-2599 ST 2088 -01 09 13 27 ST ST 1ST ST 77471 2301-2523 O RO 1037 -01 22 18 85 LA L1 RO RO3 1ST ST 77471 3201-3203 O RO 1013 -12 22 18 85 LA L6 RO RO4 1ST ST 77471 3210-3314 E RO 1013 -10 22 18 85 LA L1 RO RO4 1ST ST 77461 15056-15206 E NE 1010 -03 22 18 85 NE 1ST ST 77498 15400-15499 SL 4029 -01 22 17 26 FB SL SL1 N 1ST ST 77417 100-499 BE 1015 -01 22 18 85 LA L6 BE S 1ST ST 77417 100-499 BE 1015 -01 22 18 85 LA L6 BE W 1ST ST 77461 8300-8921 NE 1010 -01 22 18 85 NE -

2018 ANNUAL REPORT 2 2018 Annual Report
2018 ANNUAL REPORT 2 2018 Annual Report About the SRD The Strathcona Regional District (SRD) is a partnership of five municipalities and four electoral areas, which covers approximately 22,000 square kilometers (8,517 square miles). The SRD serves and provides 44,671 residents (2016 census) with a diverse range of services including water and sewerage systems, fire protection, land use planning, parks, recreation and emergency response. The Strathcona Regional District was established on February 15, 2008, as a result of the provincial government’s restructure of the Comox Strathcona Regional District. The geography of the SRD ranges from forested hills, remote inlets, picturesque villages to vibrant urban landscapes. The borders extend from the Oyster River in the south to Gold River, Sayward, Tahsis, Zeballos and Kyuquot-Nootka in the north and west, and east to Cortes Island, Quadra Island and the Discovery Islands as well as a portion of the adjacent mainland north of Powell River. 128°0'0"W 127°0'0"W 126°0'0"W 125°0'0"W 124°0'0"W Stikine Fort Nelson-Liard Kitimat-Stikine er Peace River iv R o hk at m Skeena Buckley-Nechako o Queen Charlotte Fraser H Fort George 51°0'0"N Cariboo Central Coastal Columbia Q Shuswap u Thompson e Mount Waddington Nicola e Squamish North Lillooet Okanagan n Strathcona Powell Central East River Central Kootenay Kootenay Okanagan C Comox Hope Valley Sunshine Fraser Kootenay h Coast Valley Okanagan Boundary Greater Similkameen a Alberni Nanaimo Clayoquot Vancouver Island r Cowichan lo Valley t Capital 51°0'0"N