Chronic Yersiniosis Due to Defects in the TLR5 and NOD2 Recognition Pathways
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Nh Dhhs Health Alert
THIS IS AN OFFICIAL NH DHHS HEALTH ALERT Distributed by the NH Health Alert Network [email protected] May 18, 2018, 1300 EDT (1:00 PM EDT) NH-HAN 20180518 Tickborne Diseases in New Hampshire Key Points and Recommendations: 1. Blacklegged ticks transmit at least five different infections in New Hampshire (NH): Lyme disease, Anaplasma, Babesia, Powassan virus, and Borrelia miyamotoi. 2. NH has one of the highest rates of Lyme disease in the nation, and 50-60% of blacklegged ticks sampled from across NH have been found to be infected with Borrelia burgdorferi, the bacterium that causes Lyme disease. 3. NH has experienced a significant increase in human cases of anaplasmosis, with cases more than doubling from 2016 to 2017. The reason for the increase is unknown at this time. 4. The number of new cases of babesiosis also increased in 2017; because Babesia can be transmitted through blood transfusions in addition to tick bites, providers should ask patients with suspected babesiosis whether they have donated blood or received a blood transfusion. 5. Powassan is a newer tickborne disease which has been identified in three NH residents during past seasons in 2013, 2016 and 2017. While uncommon, Powassan can cause a debilitating neurological illness, so providers should maintain an index of suspicion for patients presenting with an unexplained meningoencephalitis. 6. Borrelia miyamotoi infection usually presents with a nonspecific febrile illness similar to other tickborne diseases like anaplasmosis, and has recently been identified in one NH resident. Tests for Lyme disease do not reliably detect Borrelia miyamotoi, so providers should consider specific testing for Borrelia miyamotoi (see Attachment 1) and other pathogens if testing for Lyme disease is negative but a tickborne disease is still suspected. -

Case Definition for Non-Pestis Yersiniosis Check This Box If This Po
19-ID-03 Committee: Infectious Disease Title: Case Definition for Non-pestis Yersiniosis ☒Check this box if this position statement is an update to an existing standardized surveillance case definition: 18-ID-02 Synopsis: This position statement updates the case definition for non-pestis yersiniosis through the clarification of laboratory criteria. I. Statement of the Problem Non-pestis yersiniosis is an infection caused most commonly by the bacteria Yersinia enterocolitica or Yersinia pseudotuberculosis. These bacteria are normal intestinal and oropharyngeal colonizers of swine, and most commonly cause infections in children under 10 years of age, or adults over 70 years of age, through contaminated food. After Salmonella, Shigella, Campylobacter, and Shiga-toxin producing E. coli, th it is the 5 most commonly reported gastrointestinal bacterial illness reported through CDC Foodborne Diseases Active Surveillance Network (FoodNet), which monitors 10 sites in the United States for nine enteric pathogens transmitted through food. The increasing use of culture-independent diagnostic tests (CIDTs) in all parts of clinical medicine, and particularly for gastrointestinal illnesses, has also increased recognition of certain pathogens. Data from 2016 from FoodNet show a 29% increase in culture-confirmed and a 91% increase in CIDT-diagnosed Yersinia infections when compared to the 2013-2015 time frame. Yersinia enterocolitica and/or Yersinia pseudotuberculosis infections are reportable in 38 states, but no standard national definition exists for confirmed and probable cases. This position statement proposes a standardized case definition for non-pestis yersiniosis. II. Background and Justification Yersinia enterocolitica and Yersinia pseudotuberculosis are Gram negative rod-shaped or coccoid organisms that can be isolated from many animals and are most often transmitted to humans from undercooked or contaminated pork. -

Preventing Foodborne Illness: Yersiniosis1 Aswathy Sreedharan, Correy Jones, and Keith Schneider2
FSHN12-09 Preventing Foodborne Illness: Yersiniosis1 Aswathy Sreedharan, Correy Jones, and Keith Schneider2 What is yersiniosis? Yersiniosis is an infectious disease caused by the con- sumption of contaminated food contaminated with the bacterium Yersinia. Most foodborne infections in the US resulting from ingestion of Yersinia species are caused by Y. enterocolitica. Yersiniosis is characterized by common symptoms of gastroenteritis such as abdominal pain and mild fever (8). Most outbreaks are associated with improper food processing techniques, including poor sanitation and improper sterilization techniques by food handlers. The dis- ease is also spread by the fecal–oral route, i.e., an infected person contaminating surfaces and transmitting the disease to others by not washing his or her hands thoroughly after Figure 1. Yersinia enterocolitica bacteria growing on a Xylose Lysine going to the bathroom. The bacterium is prevalent in the Sodium Deoxycholate (XLD) agar plate. environment, enabling it to contaminate our water and Credits: CDC Public Health Image Library (ID# 6705). food systems. Outbreaks of yersiniosis have been associated with unpasteurized milk, oysters, and more commonly with What is Y. enterocolitica? consumption of undercooked dishes containing pork (8). Yersinia enterocolitica is a small, rod-shaped, Gram- Yersiniosis incidents have been documented more often negative, psychrotrophic (grows well at low temperatures) in Europe and Japan than in the United States where it is bacterium. There are approximately 60 serogroups of Y. considered relatively rare. According to the Centers for enterocolitica, of which only 11 are infectious to humans. Disease Control and Prevention (CDC), approximately Of the most common serogroups—O:3, O:8, O:9, and one confirmed Y. -

Family Outbreak of Yersiniosis TOM MARTIN,L* GORDON F
JOURNAL OF CLINICAL MICROBIOLOGY, OCt. 1982, p. 622-626 Vol. 16, No. 4 0095-1 137/82/100622-05$02.00/0 Copyright © 1982, American Society for Microbiology Family Outbreak of Yersiniosis TOM MARTIN,l* GORDON F. KASIAN,2 AND STANLEY STEAD3 Departments of Microbiology,t Pediatrics,2 and Social and Preventive Medicine,3 University of Saskatchewan, Saskatoon, Saskatchewan, Canada 57N OWO Received 1 February 1982/Accepted 24 June 1982 Yersinia enterocolitica biotype 1, serotype 0:21 was isolated from feces or rectal washings of three members of one family in northwestern Saskatchewan. The three isolates gave positive pathogenicity tests in guinea pigs with cultures grown at 22°C as inoculum. All three cases showed clinical symptoms consistent with yersiniosis. All three cases had symptoms of diarrhea and abdominal pain, and two cases had recorded fever. In two cases, appendicitis was initially suspect. One case with ileitis and peritonitis was fatal. The environmental source of the infection was not found, but river water, milk, and person-to-person spread are discussed as possible sources of the infections. The need for microbiology laboratories to culture stool specimens specifically for Y. enterocolitica, using cold-enrichment techniques is emphasized. This family outbreak of yersiniosis provides further evidence that certain biotype 1 strains of Y. enterocolitica are pathogenic. Yersinia enterocolitica is a gram-negative ba- ferred by air ambulance to University Hospital, Saska- cillus now classified as a member of the family toon. Enterobacteriaceae. A particular characteristic Two stool specimens obtained on the second and this is its to survive and sixth days after his admission to University Hospital of organism ability were negative for ova and parasites and negative for multiply at low temperatures. -

Z:\My Documents\WPDOCS\IACUC
ZOONOTIC DISEASES OF LABORATORY, AGRICULTURAL, AND WILDLIFE ANIMALS July, 2007 Michael S. Rand, DVM, DACLAM University Animal Care University of Arizona PO Box 245092 Tucson, AZ 85724-5092 (520) 626-6705 E-mail: [email protected] http://www.ahsc.arizona.edu/uac Table of Contents Introduction ............................................................................................................................................. 3 Amebiasis ............................................................................................................................................... 5 B Virus .................................................................................................................................................... 6 Balantidiasis ........................................................................................................................................ 6 Brucellosis ........................................................................................................................................ 6 Campylobacteriosis ................................................................................................................................ 7 Capnocytophagosis ............................................................................................................................ 8 Cat Scratch Disease ............................................................................................................................... 9 Chlamydiosis ..................................................................................................................................... -

61% of All Human Pathogens Are Zoonotic (Passed from Animals to Humans), and Many Are Transmitted Through Inhaling Dust Particles Or Contact with Animal Wastes
Zoonotic Diseases Fast Facts: 61% of all human pathogens are zoonotic (passed from animals to humans), and many are transmitted through inhaling dust particles or contact with animal wastes. Some of the diseases we can get from our pets may be fatal if they go undetected or undiagnosed. All are serious threats to human health, but can usually be avoided by observing a few precautions, the most effective of which is washing your hands after touching animals or their wastes. Regular visits to the veterinarian for prevention, diagnosis, and treatment of zoonotic diseases will help limit disease in your pet. Source: http://www.cdc.gov/healthypets/ Some common zoonotic diseases humans can get through their pets: Zoonotic Disease & its Effect on How Contact is Made Humans Bartonellosis (cat scratch disease) – an Bartonella bacteria are transferred to humans through infection from the bacteria Bartonella a bite or scratch. Do not play with stray cats, and henselae that causes fever and swollen keep your cat free of fleas. Always wash hands after lymph nodes. handling your cat. Capnocytophaga infection – an Capnocytophaga canimorsus is the main human infection caused by bacteria that can pathogen associated with being licked or bitten by an develop into septicemia, meningitis, infected dog and may present a problem for those and endocarditis. who are immunosuppressed. Cellulitis – a disease occurring when Bacterial organisms from the Pasteurella species live bacteria such as Pasteurella multocida in the mouths of most cats, as well as a significant cause a potentially serious infection of number of dogs and other animals. These bacteria the skin. -

Tularemia (CFSPH)
Tularemia Importance Tularemia is a zoonotic bacterial disease with a wide host range. Infections are most prevalent among wild mammals and marsupials, with periodic epizootics in Rabbit Fever, lagomorphs and rodents, but clinical cases also occur in sheep, cats and other Deerfly Fever, domesticated species. A variety of syndromes can be seen, but fatal septicemia is Meat-Cutter’s Disease common in some species. In humans, tularemia varies from a localized infection to Ohara Disease, fulminant, life-threatening pneumonia or septicemia. Francis Disease Tularemia is mainly seen in the Northern Hemisphere, where it has recently emerged or re-emerged in some areas, including parts of Europe and the Middle East. A few endemic clinical cases have also been recognized in regions where this disease Last Updated: June 2017 was not thought to exist, such as Australia, South Korea and southern Sudan. In some cases, emergence may be due to increased awareness, surveillance and/or reporting requirements; in others, it has been associated with population explosions of animal reservoir hosts, or with social upheavals such as wars, where sanitation is difficult and infected rodents may contaminate food and water supplies. Occasionally, this disease may even be imported into a country in animals. In 2002, tularemia entered the Czech Republic in a shipment of sick pet prairie dogs from the U.S. Etiology Tularemia is caused by Francisella tularensis (formerly known as Pasteurella tularensis), a Gram negative coccobacillus in the family Francisellaceae and class γ- Proteobacteria. Depending on the author, either three or four subspecies are currently recognized. F. tularensis subsp. tularensis (also known as type A) and F. -

Annual Report of Infectious Diseases 2015
Annual Report of Infectious Diseases 2015 Notes All incidence rates throughout the report are per 100,000 population based on the 2015 U.S. Census Bureau’s population data, gathered on July 6, 2016. Data for counties reporting fewer than five disease cases are not included to protect confidentiality of cases. Data for fewer than 20 reported disease cases are considered statistically unstable. Reports on HIV/AIDS, sexually transmitted infections and tuberculosis are published separately. Counts and rates for the 2015 annual report are not comparable to previous years, as changes were made to ensure case definitions match the National Notifiable Diseases list, which can be found at https://wwwn.cdc.gov/nndss/conditions/. Indiana’s reporting rule became effective December 25, 2015. While the requirements for what needs to be reported and when were updated, this report follows the reporting requirements of the previous rule. More information on the reporting rule can be found at http://www.in.gov/isdh/25366.htm. References American Academy of Pediatrics. In: Pickering LK, Baker CJ, Long SS, McMillan JA, eds. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2012. Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. Centers for Disease Control and Prevention, Atlanta, GA, 2008. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington DC: Public Health Foundation, 2015. Heyman, D.L. Control of Communicable Diseases Manual 20th ed. -

The 2014 Revised RDH Guideline Bart Currie, Royal Darwin Hospital and Menzies School of Health Research, Darwin
4 The Northern Territory Disease Control Bulletin Vol 21 No. 2 June 2014 Melioidosis: The 2014 Revised RDH Guideline Bart Currie, Royal Darwin Hospital and Menzies School of Health Research, Darwin Abstract The first reported case of melioidosis in the Northern Territory was in 1960.5 Since October Since the start of the 2009/2010 wet season the 1989 we have prospectively documented all numbers of confirmed melioidosis cases in the cases of melioidosis in the Top End. Over the 20 Top End of the Northern Territory have far years from 1 October 1989 until 30 September exceeded historical averages, with the majority 2009 there were 540 culture-confirmed cases of the increase being in the urban Darwin with 78 deaths (14%) in the Darwin Prospective region. The Darwin Prospective Melioidosis Melioidosis Study (DPMS). With heavy rains in Study commenced on 1 October 1989 and over the wet seasons from 2009-2012 case numbers the years the approach to diagnosis and rose dramatically; 91 cases (11 fatal) in 2009- treatment of melioidosis has evolved, based on 2010; 64 cases (9 fatal) in 2010-2011; and 97 the cumulative experience of Royal Darwin cases (10 fatal) in 2011-2012. In addition Hospital (RDH) clinicians and the RDH and following very heavy rains early in 2011 there Menzies laboratory staff and that of colleagues were an unprecedented 6 cases in Central elsewhere in Australia and overseas. In Australia which were considered acquired in February 2014 the RDH Melioidosis Guideline Central Australia rather than in the Top End. was revised and is presented here. -

Zoonotic Diseases of Companion Animals – by Transmission
Zoonotic Diseases Direct Contact and Fomite These diseases may be spread by bites, scratches, or direct contact with of Companion animal tissues or fluids (e.g., urine, feces, saliva). Disease transmission Animals may also occur indirectly through contact with contaminated objects or surfaces (fomites), such as cages, aquaria, bowls, or bedding. Routes of Transmission • Acariasis (mange) • Lymphocytic • Pasteurellosis • Brucellosis Choriomeningitis • Plague This handout lists • Cat Scratch Disease • Melioidosis • Q Fever potential routes of • Dermatophytosis • Monkeypox • Rabies transmission of select zoonotic diseases • Glanders • Mycobacteriosis • Rat Bite Fever between animals and humans. • Influenza • Methicillin-Resistant • Salmonellosis • Leptospirosis Staphylococcus • Sporotrichosis aureus (MRSA) • Tularemia Additional routes may occur between animals. Oral These diseases can be transmitted by ingestion of food or water contaminated with a pathogen. This typically occurs from fecal contamination from unwashed hands or soil contact. • Baylisascariasis • Echinococcosis • Toxocariasis • Campylobacteriosis • Giardiasis • Toxoplasmosis • Cryptosporidiosis • Hookworm Infection • Trichuriasis • Escherichia coli • Leptospirosis • Tularemia O157:H7 • Salmonellosis • Yersiniosis Aerosol These diseases can be transmitted through the air by droplet transfer, fluids aerosolized from an animal to a person (e.g., sneezing or cough) or by aerosolized materials which are inhaled. • Bordetella Infection • Leptospirosis • Q Fever • Cryptococcosis • Melioidosis • Tularemia • Hantavirus • Plague • Influenza • Psittacosis Vector-borne These diseases are transmitted by an arthropod vector. FLEAS TICKS TRIATOMINE • Plague • Ehrlichiosis (“kissing bugs”) • Trypanosomiasis College of Veterinary Medicine MOSQUITOES • Lyme Disease Iowa State University • Rocky Mountain (Chagas disease) • West Nile Encephalitis Ames, Iowa 50011 Spotted Fever Phone: (515) 294–7189 SAND FLIES • Tularemia FAX: (515) 294–8259 • Leishmaniasis E–mail: [email protected] Web: www.cfsph.iastate.edu © 2013. -

Healthy Brookline Volume
HEALTHY BROOKLINE VOLUME XVI Communicable Diseases in Brookline Brookline Department of Public Health 2015 ACKNOWLEDGEMENTS This report was prepared by Janelle Mellor, MPH, with support from Natalie Miller, MPH, Barbara Westley, RN, and Lynne Karsten, MPH, under the direction of Alan Balsam, PhD, MPH, Director of Public Health and Human Services in Brookline. Thanks are also due to the Division Directors at the Brookline Department of Public Health for their support and input: Lynne Karsten, MPH Patrick Maloney, MPAH Mary Minott, LICSW Patricia Norling Gloria Rudisch, MD, MPH Dawn Sibor, MEd Barbara Westley, RN A special thanks to the Brookline Advisory Council on Public Health Bruce Cohen, PhD-Chair Roberta Gianfortoni, MA Milly Krakow, PhD Cheryl Lefman, MA Patricia Maher, RN/NP, MA/MA Anthony Schlaff, MD, MPH Support and data were also provided by: Susan Soliva, MPH, Massachusetts Department of Public Health The Healthy Brookline Chartbooks represent a partnership with a variety of funding sources: Beth Israel Deaconess Medical Center Brigham & Women’s Hospital Children’s Hospital Farnsworth Trust Tufts Medical Center St. Elizabeth’s Medical Center Blue Cross Blue Shield of Massachusetts Brookline Community Foundation Harvard Pilgrim Health Care Foundation Tufts Health Plan We thank them all for their generous support. Table of Contents Section 1: Communicable Disease Surveillance and Reporting .................................................................. 1 Surveillance and Reporting ...................................................................................................................... -
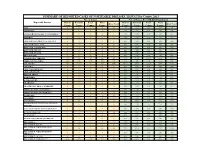
Individual Year Summary of Reported Cases for 2013
SUMMARY OF REPORTED CASES OF NOTIFIABLE DISEASES, HAWAI`I by County, 2013 No. of Cases No. of Cases Per 100,000 Population Reportable Diseases Hawaii Honolulu Kauai Maui Hawaii Honolulu Kauai Maui State Total State Total County County County County County County County County AIDS* 9 46 1 9 66 4.70 4.67 1.44 5.59 4.69 AMEBIASIS * 2 1 0 1 4 1.04 0.10 0.00 0.62 0.28 ANGIOSTRONGYLIASIS, CANTONENSIS*^ 3 0 0 0 3 1.57 0.00 0.00 0.00 0.21 ANTHRAX 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 ARBOVIRUSES, GROUP A & GROUP B 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 ARENAVIRUSES, LASSA 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 BOTULISM, FOODBORNE 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 BOTULISM, INFANT 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 BOTULISM, WOUND 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 BRUCELLOSIS 1 0 0 0 1 0.52 0.00 0.00 0.00 0.07 CAMPYLOBACTERIOSIS 142 521 70 88 825 74.12 52.85 100.54 54.63 58.59 CHIKUNGUNYA VIRUS N/R N/R N/R N/R N/R N/A N/R N/R N/R N/R CHLAMYDIA 768 5185 161 523 6640 400.87 526.00 231.24 324.65 471.58 CHOLERA 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 CRYPTOSPORIDIOSIS 0 1 0 0 1 0.00 0.10 0.00 0.00 0.07 CYCLOSPORIASIS 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 DENGUE FEVER 1 6 0 3 10 0.52 0.61 0.00 1.86 0.71 DIPHTHERIA 0 0 0 0 0 0.00 0.00 0.00 0.00 0.00 E.