Annual Report of Infectious Diseases 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Nh Dhhs Health Alert
THIS IS AN OFFICIAL NH DHHS HEALTH ALERT Distributed by the NH Health Alert Network [email protected] May 18, 2018, 1300 EDT (1:00 PM EDT) NH-HAN 20180518 Tickborne Diseases in New Hampshire Key Points and Recommendations: 1. Blacklegged ticks transmit at least five different infections in New Hampshire (NH): Lyme disease, Anaplasma, Babesia, Powassan virus, and Borrelia miyamotoi. 2. NH has one of the highest rates of Lyme disease in the nation, and 50-60% of blacklegged ticks sampled from across NH have been found to be infected with Borrelia burgdorferi, the bacterium that causes Lyme disease. 3. NH has experienced a significant increase in human cases of anaplasmosis, with cases more than doubling from 2016 to 2017. The reason for the increase is unknown at this time. 4. The number of new cases of babesiosis also increased in 2017; because Babesia can be transmitted through blood transfusions in addition to tick bites, providers should ask patients with suspected babesiosis whether they have donated blood or received a blood transfusion. 5. Powassan is a newer tickborne disease which has been identified in three NH residents during past seasons in 2013, 2016 and 2017. While uncommon, Powassan can cause a debilitating neurological illness, so providers should maintain an index of suspicion for patients presenting with an unexplained meningoencephalitis. 6. Borrelia miyamotoi infection usually presents with a nonspecific febrile illness similar to other tickborne diseases like anaplasmosis, and has recently been identified in one NH resident. Tests for Lyme disease do not reliably detect Borrelia miyamotoi, so providers should consider specific testing for Borrelia miyamotoi (see Attachment 1) and other pathogens if testing for Lyme disease is negative but a tickborne disease is still suspected. -

Case Definition for Non-Pestis Yersiniosis Check This Box If This Po
19-ID-03 Committee: Infectious Disease Title: Case Definition for Non-pestis Yersiniosis ☒Check this box if this position statement is an update to an existing standardized surveillance case definition: 18-ID-02 Synopsis: This position statement updates the case definition for non-pestis yersiniosis through the clarification of laboratory criteria. I. Statement of the Problem Non-pestis yersiniosis is an infection caused most commonly by the bacteria Yersinia enterocolitica or Yersinia pseudotuberculosis. These bacteria are normal intestinal and oropharyngeal colonizers of swine, and most commonly cause infections in children under 10 years of age, or adults over 70 years of age, through contaminated food. After Salmonella, Shigella, Campylobacter, and Shiga-toxin producing E. coli, th it is the 5 most commonly reported gastrointestinal bacterial illness reported through CDC Foodborne Diseases Active Surveillance Network (FoodNet), which monitors 10 sites in the United States for nine enteric pathogens transmitted through food. The increasing use of culture-independent diagnostic tests (CIDTs) in all parts of clinical medicine, and particularly for gastrointestinal illnesses, has also increased recognition of certain pathogens. Data from 2016 from FoodNet show a 29% increase in culture-confirmed and a 91% increase in CIDT-diagnosed Yersinia infections when compared to the 2013-2015 time frame. Yersinia enterocolitica and/or Yersinia pseudotuberculosis infections are reportable in 38 states, but no standard national definition exists for confirmed and probable cases. This position statement proposes a standardized case definition for non-pestis yersiniosis. II. Background and Justification Yersinia enterocolitica and Yersinia pseudotuberculosis are Gram negative rod-shaped or coccoid organisms that can be isolated from many animals and are most often transmitted to humans from undercooked or contaminated pork. -

Invasive Group a Streptococcal Disease Communicable Disease Control Unit
Public Health and Primary Health Care Communicable Disease Control 4th Floor, 300 Carlton St, Winnipeg, MB R3B 3M9 T 204 788-6737 F 204 948-2040 www.manitoba.ca November, 2015 Re: Streptococcal Invasive Disease (Group A) Reporting and Case Investigation Reporting of Streptococcal invasive disease (Group A) (Streptococcus pyogenes) is as follows: Laboratory: All specimens isolated from sterile sites (refer to list below) that are positive for S. pyogenes are reportable to the Public Health Surveillance Unit by secure fax (204-948-3044). Health Care Professional: Probable (clinical) cases of Streptococcal invasive disease (Group A) are reportable to the Public Health Surveillance Unit using the Clinical Notification of Reportable Diseases and Conditions form (http://www.gov.mb.ca/health/publichealth/cdc/protocol/form13.pdf) ONLY if a positive lab result is not anticipated (e.g., poor or no specimen taken, person has recovered). Cooperation in Public Health investigation (when required) is appreciated. Regional Public Health or First Nations Inuit Health Branch (FNIHB): Cases will be referred to Regional Public Health or FNIHB. Completion and return of the Communicable Disease Control Investigation Form is generally not required, unless otherwise directed by a Medical Officer of Health. Sincerely, “Original Signed By” “Original Signed By” Richard Baydack, PhD Carla Ens, PhD Director, Communicable Disease Control Director, Epidemiology & Surveillance Public Health and Primary Health Care Public Health and Primary Health Care Manitoba Health, Healthy Living and Seniors Manitoba Health, Healthy Living and Seniors The sterile and non-sterile sites listed below represent commonly sampled body sites for the purposes of diagnosis, but the list is not exhaustive. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Toxic Shock-Like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection
Henry Ford Hospital Medical Journal Volume 37 Number 2 Article 5 6-1989 Toxic Shock-like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection Thomas J. Connolly Donald J. Pavelka Eugene F. Lanspa Thomas L. Connolly Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Connolly, Thomas J.; Pavelka, Donald J.; Lanspa, Eugene F.; and Connolly, Thomas L. (1989) "Toxic Shock- like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection," Henry Ford Hospital Medical Journal : Vol. 37 : No. 2 , 69-72. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol37/iss2/5 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Toxic Shock-like Syndrome Associated with Necrotizing Streptococcus Pyogenes Infection Thomas J. Connolly,* Donald J. Pavelka, MD,^ Eugene F. Lanspa, MD, and Thomas L. Connolly, MD' Two patients with toxic shock-like syndrome are presented. Bolh patients had necrotizing cellulitis due to Streptococcus pyogenes, and both patients required extensive surgical debridement. The association of Streptococcus pyogenes infection and toxic shock-like syndrome is discussed. (Henry Ford Hosp MedJ 1989:37:69-72) ince 1978, toxin-producing strains of Staphylococcus brought to the emergency room where a physical examination revealed S aureus have been implicated as the cause of the toxic shock a temperature of 40.9°C (I05.6°F), blood pressure of 98/72 mm Hg, syndrome (TSS), which is characterized by fever and rash and respiration of 36 breaths/min, and a pulse of 72 beats/min. -
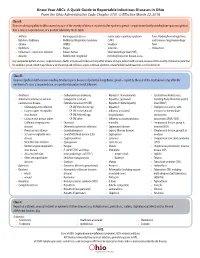
The Know Your Abcs: a Quick Guide to Reportable Infectious Diseases
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective March 22, 2018 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Meningococcal disease • Severe acute respiratory syndrome fever, Marburg hemorrhagic fever, • Botulism, foodborne • Middle East Respiratory Syndrome (SARS) and Crimean-Congo hemorrhagic • Cholera (MERS) • Smallpox fever • Diphtheria • Plague • Tularemia • Yellow fever • Influenza A – novel virus infection • Rabies, human • Viral hemorrhagic fever (VHF), • Measles • Rubella (not congenital) including Ebola virus disease, Lassa Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis C (non-perinatal) • Spotted Fever Rickettsiosis, • Arboviral neuroinvasive and non- carbapenem-resistant • Hepatitis C (perinatal) including Rocky Mountain spotted neuroinvasive disease: Enterobacteriaceae (CP-CRE) • Hepatitis D (delta hepatitis) fever (RMSF) • Chikungunya virus infection • CP-CRE Enterobacter spp. • Hepatitis E • Staphylococcus aureus, with • Eastern equine encephalitis • CP-CRE Escherichia coli • Influenza-associated resistance or intermediate virus disease • CP-CRE Klebsiella spp. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

VENEREAL DISEASES in ETHIOPIA Survey and Recommendations THORSTEIN GUTHE, M.D., M.P.H
Bull. World Hlth Org. 1949, 2, 85-137 10 VENEREAL DISEASES IN ETHIOPIA Survey and Recommendations THORSTEIN GUTHE, M.D., M.P.H. Section on Venereal Diseases World Health Organization Page 1. Prevalent diseases . 87 1.1 Historical .............. 87 1.2 Distribution.............. 88 2. Syphilis and related infections . 89 2.1 Spread factors . 89 2.2 Nature of syphilis . 91 2.3 Extent of syphilis problem . 98 2.4 Other considerations . 110 3. Treatment methods and medicaments . 114 3.1 Ancient methods of treatment . 114 3.2 Therapy and drugs . 115 4. Public-health organization. 116 4.1 Hospital facilities . 117 4.2 Laboratory facilities . 120 4.3 Personnel .............. 121 4.4 Organizational structure . 122 4.5 Legislation.............. 124 5. Recommendations for a venereal-disease programme . 124 5.1 General measures. ........... 125 5.2 Personnel, organization and administration . 126 5.3 Collection of data . 127 5.4 Diagnostic and laboratory facilities . 129 5.5 Treatment facilities . 130 5.6 Case-finding, treatment and follow-up . 131 5.7 Budget. ......... ... 134 6. Summary and conclusions . 134 References . 136 In spite of considerable handicaps, valuable developments in health took place in Ethiopia during the last two decades. This work was abruptly arrested by the war, and the fresh start necessary on the liberation of the country emphasized that much health work still remains to be done. A realistic approach to certain disease-problems and the necessity for compe- tent outside assistance to tackle such problems form the basis for future work. The accomplishments of the Ethiopian Government in the limited time since the war bode well for the future. -
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Leprosy in Two Patients with Relapsing Remitting Multiple Sclerosis Treated with Fingolimod Alfred Balasa, M.D.1 and George J
Leprosy in Two Patients with Relapsing Remitting Multiple Sclerosis Treated with Fingolimod Alfred Balasa, M.D.1 and George J. Hutton, M.D.2 1 Department of Pediatrics, Section of Pediatric Neurology and Developmental Neuroscience; 2 Department of Neurology; Baylor College of Medicine, Houston, Texas Background A B Leprosy (Hansen’s disease) is a chronic infection caused by Mycobacterium leprae. The disease develops over months to years and may cause extensive damage to the skin and peripheral nervous system. A B In multiple sclerosis (MS), fingolimod treatment is Figure 3. Immune response in the polar clinical forms of leprosy. known to increase the risk for infections. There is one prior reported case of leprosy while on [A] In tuberculoid leprosy (TT) patients, the innate immune response is activated by M. leprae through toll-like receptors (TLR2/1). IL-15 fingolimod for relapsing remitting multiple sclerosis stimulates the vitamin D-dependent antimicrobial program in macrophages and inhibits phagocytosis of mycobacteria. These events (RRMS). We report two further cases of leprosy in promote a Th1 T-cell cytokine response (IFN-γ, IL-2, TNF, and IL-15) that contains the infection in well-formed granulomas, and a Th17 patients with RRMS and treated with fingolimod. C D response (IL-17A, IL-17F, IL-21 and IL-22) that leads to tissue inflammation and destruction, neutrophil recruitment, macrophage activation, and enhancement of Th1 effector cells. [B] In lepromatous leprosy (LL) patients, IL-4, IL-10, leukocyte immunoglobulin-like receptor subfamily A member 2 (LILRA2), and oxidized phospholipids inhibit TLR2/1-induced cytokine responses but preserve IL-10 release. -

Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis