Immunological Aspects of Leprosy with Special Reference to Autoimmune Diseases 0
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -
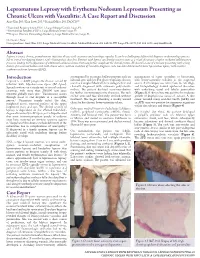
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Leprosy in Two Patients with Relapsing Remitting Multiple Sclerosis Treated with Fingolimod Alfred Balasa, M.D.1 and George J
Leprosy in Two Patients with Relapsing Remitting Multiple Sclerosis Treated with Fingolimod Alfred Balasa, M.D.1 and George J. Hutton, M.D.2 1 Department of Pediatrics, Section of Pediatric Neurology and Developmental Neuroscience; 2 Department of Neurology; Baylor College of Medicine, Houston, Texas Background A B Leprosy (Hansen’s disease) is a chronic infection caused by Mycobacterium leprae. The disease develops over months to years and may cause extensive damage to the skin and peripheral nervous system. A B In multiple sclerosis (MS), fingolimod treatment is Figure 3. Immune response in the polar clinical forms of leprosy. known to increase the risk for infections. There is one prior reported case of leprosy while on [A] In tuberculoid leprosy (TT) patients, the innate immune response is activated by M. leprae through toll-like receptors (TLR2/1). IL-15 fingolimod for relapsing remitting multiple sclerosis stimulates the vitamin D-dependent antimicrobial program in macrophages and inhibits phagocytosis of mycobacteria. These events (RRMS). We report two further cases of leprosy in promote a Th1 T-cell cytokine response (IFN-γ, IL-2, TNF, and IL-15) that contains the infection in well-formed granulomas, and a Th17 patients with RRMS and treated with fingolimod. C D response (IL-17A, IL-17F, IL-21 and IL-22) that leads to tissue inflammation and destruction, neutrophil recruitment, macrophage activation, and enhancement of Th1 effector cells. [B] In lepromatous leprosy (LL) patients, IL-4, IL-10, leukocyte immunoglobulin-like receptor subfamily A member 2 (LILRA2), and oxidized phospholipids inhibit TLR2/1-induced cytokine responses but preserve IL-10 release. -

Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe Between 2009 and 2018
microorganisms Article Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018 Anna Beltrame 1,* , Gianfranco Barabino 2, Yiran Wei 2, Andrea Clapasson 2, Pierantonio Orza 1, Francesca Perandin 1 , Chiara Piubelli 1 , Geraldo Badona Monteiro 1, Silvia Stefania Longoni 1, Paola Rodari 1 , Silvia Duranti 1, Ronaldo Silva 1 , Veronica Andrea Fittipaldo 3 and Zeno Bisoffi 1,4 1 Department of Infectious, Tropical Diseases and Microbiology, I.R.C.C.S. Sacro Cuore Don Calabria Hospital, Via Sempreboni 5, 37024 Negrar di Valpolicella, Italy; [email protected] (P.O.); [email protected] (F.P.); [email protected] (C.P.); [email protected] (G.B.M.); [email protected] (S.S.L.); [email protected] (P.R.); [email protected] (S.D.); [email protected] (R.S.); zeno.bisoffi@sacrocuore.it (Z.B.) 2 Dermatological Clinic, National Reference Center for Hansen’s Disease, Ospedale Policlinico San Martino, Sistema Sanitario Regione Liguria, Istituto di Ricovero e Cura a Carattere Scientifico per l’Oncologia, Largo Rosanna Benzi 10, 16132 Genoa, Italy; [email protected] (G.B.); [email protected] (Y.W.); [email protected] (A.C.) 3 Oncology Department, Mario Negri Institute for Pharmacological Research I.R.C.C.S., Via Giuseppe La Masa 19, 20156 Milano, Italy; vafi[email protected] 4 Department of Diagnostic and Public Health, University of Verona, P.le L. A. Scuro 10, 37134 Verona, Italy * Correspondence: [email protected]; Tel.: +39-045-601-4748 Received: 30 June 2020; Accepted: 23 July 2020; Published: 24 July 2020 Abstract: Leprosy is a chronic neglected infectious disease that affects over 200,000 people each year and causes disabilities in more than four million people in Asia, Africa, and Latin America. -

NAIL CHANGES in RECENT and OLD LEPROSY PATIENTS José M
NAIL CHANGES IN RECENT AND OLD LEPROSY PATIENTS José M. Ramos,1 Francisco Reyes,2 Isabel Belinchón3 1. Department of Internal Medicine, Hospital General Universitario de Alicante, Alicante, Spain; Associate Professor, Department of Medicine, Miguel Hernández University, Spain; Medical-coordinator, Gambo General Rural Hospital, Ethiopia 2. Medical Director, Gambo General Rural Hospital, Ethiopia 3. Department of Dermatology, Hospital General Universitario de Alicante, Alicante, Spain; Associate Professor, Department of Medicine, Miguel Hernández University, Spain Disclosure: No potential conflict of interest. Received: 27.09.13 Accepted: 21.10.13 Citation: EMJ Dermatol. 2013;1:44-52. ABSTRACT Nails are elements of skin that can often be omitted from the dermatological assessment of leprosy. However, there are common nail conditions that require special management. This article considers nail presentations in leprosy patients. General and specific conditions will be discussed. It also considers the common nail conditions seen in leprosy patients and provides a guide to diagnosis and management. Keywords: Leprosy, nails, neuropathy, multibacillary leprosy, paucibacillary leprosy, acro-osteolysis, bone atrophy, type 2 lepra reaction, anonychia, clofazimine, dapsone. INTRODUCTION Leprosy can cause damage to the nails, generally indirectly. There are few reviews about the Leprosy is a chronic granulomatous infection affectation of the nails due to leprosy. Nails are caused by Mycobacterium leprae, known keratin-based elements of the skin structure that since ancient times and with great historical are often omitted from the dermatological connotations.1 This infection is not fatal but affects assessment of leprosy. However, there are the skin and peripheral nerves. The disease causes common nail conditions that require diagnosis cutaneous lesions, skin lesions, and neuropathy, and management. -

Tuberous Sclerosis Complex: in Mother and Daughter
Case Report DOI: 10.18231/2455-6769.2017.0011 Tuberous sclerosis complex: in mother and daughter Krishnendra Varma1, Prashant Harit2,* 1Professor & HOD, 2Junior Resident, Dept. of Dermatology, Venereology & Leprosy, RD Gardi Medical College, Ujjain, Madhya Pradesh *Corresponding Author: Email: [email protected] Abstract Tuberous sclerosis (TS) is an autosomal dominant multisystem disorder characterized by a triad of epilepsy, mental retardation and adenoma sebaceum with multisystem involvement including kidney, brain, skin, eyes, heart and lung. The most frequently affected organs in TSC are kidney and brain. We described TSC in mother and her daughter. We emphasized the importance of Dermatological and Radiological examination to find asymptomatic renal and Central Nervous System Lesion. Keywords: Tuberous sclerosis, Hamartoma, Angiofibroma. Introduction intellectually she was alright and her IQ test was Tuberous sclerosis also known as Bourneville's normal. disease; Pringle disease; EPILOIA (epilepsy, low On investigation Histopathology of facial and intelligence, adenoma sebaceum).(1) lumbosacral region were consistent with angiofibroma The name is derived from Latin tuber and the and shagreen patch respectively. Ultrasonography Greek skleros (hard). Tubers are potato-like nodules of whole abdomen shows multiple renal angiomyolipoma glial proliferation and are characteristic central nervous (Fig. 3). Her CT KUB (Kidney, Ureter and Bladder) system lesions of tuberous sclerosis complex. were consistent with angiomyolipoma (Fig. 4). MRI Its Inheritance is autosomal dominant with variable Brain showed presence of calcified tubers in foramen of expression and approximately 60-70% of tuberous monro, Non calcified subependymal nodule in bilateral sclerosis complex (TSC) is attributable to a new peritrigonal area, cortical tuber was also found in left mutation. -

ORIGINAL ARTICLE CD5+ B Cells Ratio in Lepromatous Leprosy
Ilhan F. Et Al: Cd5+ B Cells Ratio In Lepromatous Leprosy JOURNAL OF CLINICAL AND DIAGNOSTIC RESEARCH How to cite this article: Ilhan F, Cicek D, Gödekmerdan A, Tahran G, Bulut V. CD5+ B CELLS RATIO IN LEPROMATOUS LEPROSY. Journal of Clinical and Diagnostic Research [serial online] 2007 June [cited: 2007June 4]; 3:138-142. Available from http://www.jcdr.net/back_issues.asp?issn=0973- 709x&year=2007&month=June&volume=1&issue=3&page=138-142&id=54 137 Journal of Clinical and Diagnostic Research. 2007June; 1(3)138-141 Ilhan F. Et Al: Cd5+ B Cells Ratio In Lepromatous Leprosy ORIGINAL ARTICLE CD5+ B Cells Ratio In Lepromatous Leprosy Ilhan F, Cicek D, Gödekmerdan A, Tahran G, Bulut V ABSTRACT Objective Leprosy is a unique infectious disease, which is chronic and related to cellular immunity. It was known that who have a defect on cellular immunity, they are susceptible to M. leprae infection. CD19+CD5+ B cells are currently defined as B1 cells and they produce polyreactive antibodies. The aim of this study was to investigate the presence and the possible effect of natural antibody producing CD19+CD5+ B cells in leprosy disease patients. Materials and Methods We investigated B lymphocyte subset and total B and B1 cells by flow cytometry in 40 patients with lepromatous leprosy and 40 healthy controls. Results Compared to the healthy controls, both CD19+CD5- conventional B cells and CD19+CD5+ B1 cells subsets were found to be higher in leprosy patients group. Conclusion The observed significant increases in CD19+CD5- and CD19+CD5+ B1 cells subsets in patients with lepromatous leprosy suggests that B1 lymphocytes known predisposed to autoimmunity may have a role on disorganized protective immune response in Lepromatous leprosy. -

Annual Report of Infectious Diseases 2015
Annual Report of Infectious Diseases 2015 Notes All incidence rates throughout the report are per 100,000 population based on the 2015 U.S. Census Bureau’s population data, gathered on July 6, 2016. Data for counties reporting fewer than five disease cases are not included to protect confidentiality of cases. Data for fewer than 20 reported disease cases are considered statistically unstable. Reports on HIV/AIDS, sexually transmitted infections and tuberculosis are published separately. Counts and rates for the 2015 annual report are not comparable to previous years, as changes were made to ensure case definitions match the National Notifiable Diseases list, which can be found at https://wwwn.cdc.gov/nndss/conditions/. Indiana’s reporting rule became effective December 25, 2015. While the requirements for what needs to be reported and when were updated, this report follows the reporting requirements of the previous rule. More information on the reporting rule can be found at http://www.in.gov/isdh/25366.htm. References American Academy of Pediatrics. In: Pickering LK, Baker CJ, Long SS, McMillan JA, eds. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2012. Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. Centers for Disease Control and Prevention, Atlanta, GA, 2008. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington DC: Public Health Foundation, 2015. Heyman, D.L. Control of Communicable Diseases Manual 20th ed. -

Skin Care Physicians Insight on Epidemiological Patterns, Diagnosis and Treatment Modalities for Female Pattern Hair Loss
International Journal of Research in Dermatology Kilaru KR et al. Int J Res Dermatol. 2021 Jan;7(1):11-17 http://www.ijord.com DOI: https://dx.doi.org/10.18203/issn.2455-4529.IntJResDermatol20204997 Original Research Article Skin care physicians insight on epidemiological patterns, diagnosis and treatment modalities for female pattern hair loss Krishna Rajesh Kilaru*, Suhasini Attada, Pooja Munnangi, Manogna Chowdary Kilaru Department of Dermatology, Venereology and Leprosy, NRI Medical College, Chinakakani, Guntur, Andhra Pradesh, India Received: 02 November 2020 Revised: 10 November 2020 Accepted: 11 November 2020 *Correspondence: Dr. Krishna Rajesh Kilaru, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Female pattern hair loss (FPHL) is a common cause of hair loss in women characterized by a diffuse reduction in hair density over the crown and frontal scalp with retention of the frontal hairline. The underlying pathophysiology is multifactorial. There are no universally agreed treatment guidelines available. The objective of the study was to understand the diagnosis and treatment pattern of female pattern hair loss and the role of minoxidil topical formulation and its combination in the management of FPHL. Methods: Predesigned questionnaire on FPHL was prepared based on review of literature and was filled by 80 consultant dermatologists. Recorded data was statistically analyzed. Results: Common age of onset of FPHL was between 20 to 30 years. -

Dermoscopic Characteristics of Newly Diagnosed Hansen's Disease
ORIGINAL ARTICLE Dermoscopic Characteristics of Newly Diagnosed Hansen’s Disease: A Prospective Descriptive Study Roshni Kakitha1, Sreedevi Ambujam2 ABSTRACT Background: Dermoscopy is an in vivo, noninvasive technique that is a one-time investment, easy to operate, and permits the visualization of morphologic features of the skin that are not visible to the naked eye. Its usage as a diagnostic tool in Leprosy/Hansen’s Disease (HD), an infiltrative disease is largely unexplored worldwide. Aims: To study dermoscopic features of newly diagnosed leprosy, and to compare these features with those of normal skin. Materials and methods: Prospective descriptive study conducted in 33 consecutive newly diagnosed leprosy patients and matched controls over a period of 18 months. Results: Changes in skin pattern were particularly discernible with white light. Yellow dots, white dots, hair density, and pigmentary network were appreciated using polarized light and ultraviolet light highlighted scales. All leprosy patches showed pigmentary dilution. Twenty-two of the 33 patients showed skin pattern loss both in the center and margin of the lesion. Perilesional skin showed normal pattern. Loss of skin pattern in comparison with the control group showed statistically significant difference. Ten patients showed patchy loss of skin pattern probably indicating early changes. Lesional skin but not the control skin showed a statistically significant reduction in white dots and hair density. Affected skin showed insignificant reduction in the number of yellow dots, i.e., sebaceous glands. Scaling was present in the margin and absent in the center, perilesional, and control skin. Limitations: The dermoscope that had been provided for the study did not have advanced features affecting the image quality. -

Infection Control Through the Ages
American Journal of Infection Control 40 (2012) 35-42 Contents lists available at ScienceDirect American Journal of Infection Control American Journal of Infection Control journal homepage: www.ajicjournal.org Major article Infection control through the ages Philip W. Smith MD a,*, Kristin Watkins MBA b, Angela Hewlett MD a a Division of Infectious Diseases, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE b Center for Preparedness Education, College of Public Health, University of Nebraska Medical Center, Omaha, NE Key Words: To appreciate the current advances in the field of health care epidemiology, it is important to understand History the history of hospital infection control. Available historical sources were reviewed for 4 different Hospitals historical time periods: medieval, early modern, progressive, and posteWorld War II. Hospital settings Nosocomial for the time periods are described, with particular emphasis on the conditions related to hospital infections. Copyright Ó 2012 by the Association for Professionals in Infection Control and Epidemiology, Inc. Published by Elsevier Inc. All rights reserved. Approximately 1.7 million health careeassociated infections One of the few public health measures was the collection of (HAIs) occur in the United States each year.1 Hospital infection bodies of plague victims. The bodies were left in the street to be control programs are nearly universal in developed nations and have picked up by carts and placed in mass graves outside of town.3,4 significantly lowered the risk of acquiring a HAI since their inception Other infection control measures included hanging people who in the mid 20th century. As we debate the preventability of HAIs, as wandered in from an epidemic region into an uninfected area, well as the ethical and logistic aspects of patient safety, it is impor- shutting up plague victims in their homes, and burning clothing tant to recall the historical context of hospital infection control. -

Chronic Care for Neglected Infectious Diseases: Leprosy/ Hansen's Disease, Lymphatic Filariasis, Trachoma, and Chagas Disease
Chronic Care for Neglected Infectious Diseases: Leprosy/ Hansen’s Disease, Lymphatic Filariasis, Trachoma, and Chagas Disease A Guide for Morbidity Management and Disability Prevention for Primary Health Care Services p Chronic Care for Neglected Infectious Diseases: Leprosy/ Hansen’s Disease, Lymphatic Filariasis, Trachoma, and Chagas Disease A Guide for Morbidity Management and Disability Prevention for Primary Health Care Services Washington, D.C., 2021 Chronic Care for Neglected Infectious Diseases: Leprosy/Hansen’s Disease, Lymphatic Filariasis, Trachoma, and Chagas Disease © Pan American Health Organization, 2021 ISBN: 978-92-75-12250-1 eISBN: 978-92-75-12251-8 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial- ShareAlike 3.0 IGO license (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/ igo). Under the terms of this license, this work may be copied, redistributed, and adapted for non-commercial purposes, provided the new work is issued using the same or equivalent Creative Commons license and it is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that the Pan American Health Organization (PAHO) endorses any specific organization, product, or service. Use of the PAHO logo is not permitted. Adaptations: If this work is adapted, the following disclaimer should be added along with the suggested citation: “This is an adaptation of an original work by the Pan American Health Organization (PAHO). Views and opinions expressed in the adaptation are the sole responsibility of the author(s) of the adaptation and are not endorsed by PAHO.” Translation: If this work is translated, the following disclaimer should be added along with the suggested citation: “This translation was not created by the Pan American Health Organization (PAHO).