University of Illinois at Chicago, December 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -
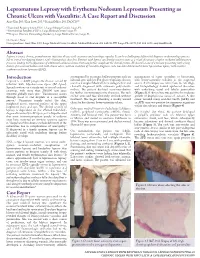
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Leprosy in Two Patients with Relapsing Remitting Multiple Sclerosis Treated with Fingolimod Alfred Balasa, M.D.1 and George J
Leprosy in Two Patients with Relapsing Remitting Multiple Sclerosis Treated with Fingolimod Alfred Balasa, M.D.1 and George J. Hutton, M.D.2 1 Department of Pediatrics, Section of Pediatric Neurology and Developmental Neuroscience; 2 Department of Neurology; Baylor College of Medicine, Houston, Texas Background A B Leprosy (Hansen’s disease) is a chronic infection caused by Mycobacterium leprae. The disease develops over months to years and may cause extensive damage to the skin and peripheral nervous system. A B In multiple sclerosis (MS), fingolimod treatment is Figure 3. Immune response in the polar clinical forms of leprosy. known to increase the risk for infections. There is one prior reported case of leprosy while on [A] In tuberculoid leprosy (TT) patients, the innate immune response is activated by M. leprae through toll-like receptors (TLR2/1). IL-15 fingolimod for relapsing remitting multiple sclerosis stimulates the vitamin D-dependent antimicrobial program in macrophages and inhibits phagocytosis of mycobacteria. These events (RRMS). We report two further cases of leprosy in promote a Th1 T-cell cytokine response (IFN-γ, IL-2, TNF, and IL-15) that contains the infection in well-formed granulomas, and a Th17 patients with RRMS and treated with fingolimod. C D response (IL-17A, IL-17F, IL-21 and IL-22) that leads to tissue inflammation and destruction, neutrophil recruitment, macrophage activation, and enhancement of Th1 effector cells. [B] In lepromatous leprosy (LL) patients, IL-4, IL-10, leukocyte immunoglobulin-like receptor subfamily A member 2 (LILRA2), and oxidized phospholipids inhibit TLR2/1-induced cytokine responses but preserve IL-10 release. -

Pilar Sheath Acanthoma Presenting As a Nevus
Letter to Editor Pilar Sheath Acanthoma Presenting as a Nevus Sir, Pilar sheath acanthoma (PSA) is a rare benign follicular neoplasm, which was first described by Mehregan and Brownstein in 1978.[1] PSA usually presents as an asymptomatic, flesh colored papule with a central opening localized at the lower lip with exceptional presentations such as ear lobe, postauricular region, or cheek.[1‑3] A 42‑year‑old female referred with a solitary, slow‑growing nodular lesion at the upper lip region for 6 months. Physical exam revealed a 4 mm, pink‑brown colored nodule with a central opening [Figure 1]. Under clinical prediagnosis of melanocytic nevus, an excisional biopsy Figure 1: Physical examination of the nodule in the upper lip region was performed. In a microscopic examination, a cystic cavity that communicated with surface epidermis has been observed. The wall of the cystic cavity was composed of solid tumor islands extending in the deep dermis [Figure 2]. The cavity was lined with stratified squamous epithelium filled with keratin [Figure 3]. PSA is an uncommon, benign follicular tumor occurring in the faces of middle‑aged and elderly patients. These lesions can present at any location such as cheek, ear lobe on the head, and neck. In our case, a 42‑year‑old female was presented with a pink‑brown colored nodular lesion opening at the upper lip region. The differential diagnosis includes trichofolliculoma and dilated pore of Winer. Trichofolliculomas contain many seconder hair follicles Figure 2: A central cavity with keratin in the dermis which is continuous radiating from the wall of the primary follicle with outer with the surface epithelium (H and E, ×40) and inner root sheaths in a well‑formed stroma which are absent in PSA. -

Immunological Aspects of Leprosy with Special Reference to Autoimmune Diseases 0
'Bull. Org. mond. Sante 1969, 41, 793-804 Bull. Wld Hlth Org. Immunological Aspects of Leprosy with Special Reference to Autoimmune Diseases 0. WAGER1 Leprosy, particularly lepromatous leprosy, is associated with a multitude of (auto) immune aberrations, and its clinical features also have much in common with the collagen diseases. Immunopathological studies of the 2 groups of diseases may thus elucidate the basic mechanisms of both. The reported evidencefor a genetically determined hyporeactivity ofcell-mediated (CM) immunity in lepromatous subjects is reviewed; most, but not all, of the findings fit such a hypothesis well. The possibility remains that the observed hyporeactivities may be secondary to direct effects of Mycobacterium leprae. Evidencefor a general hyperreactivity of the antibody-mediated (AM) immunity in lepromatous leprosy is then reviewed and considered to be fragmentary. The concept and general criteria of autoimmunity are discussed briefly and the high incidence in lepromatous leprosy of various (auto)immune aberrations, resembling those in systemic lupus erythematosus (SLE) and in rheumatoid arthritis is reviewed. Although autoantibodies are not likely to be directly deleterious to the host, immune complexes containing autoantibodies may be pathogenic. Mixed cryoimmunoglobulins, consisting of 2 (IgG-IgM or IgG-IgA) or 3 immunoglo-. bulins, and occasionally also containing measurable amounts of complement components, have recently been encountered in SLE and its variants and also in a number of microbial diseases with autoimmune features (syphilis, streptococcal nephritis and endocarditis, mononucleosis, Mycoplasma pneumoniae pneumonia). They may represent circulating immune complexes, analogous to the IgM (IgA) rheumatoid factors in combination with their IgG reactants. In leprosy also, the existence of pathogenic immune complexes is indirectly suggested by mixed cryoglobulinemia andfurther by a number of other features reviewed in this article. -

What Are Basal and Squamous Cell Skin Cancers?
cancer.org | 1.800.227.2345 About Basal and Squamous Cell Skin Cancer Overview If you have been diagnosed with basal or squamous cell skin cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Are Basal and Squamous Cell Skin Cancers? Research and Statistics See the latest estimates for new cases of basal and squamous cell skin cancer and deaths in the US and what research is currently being done. ● Key Statistics for Basal and Squamous Cell Skin Cancers ● What’s New in Basal and Squamous Cell Skin Cancer Research? What Are Basal and Squamous Cell Skin Cancers? Basal and squamous cell skin cancers are the most common types of skin cancer. They start in the top layer of skin (the epidermis), and are often related to sun exposure. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Cancer starts when cells in the body begin to grow out of control. Cells in nearly any part of the body can become cancer cells. To learn more about cancer and how it starts and spreads, see What Is Cancer?1 Where do skin cancers start? Most skin cancers start in the top layer of skin, called the epidermis. There are 3 main types of cells in this layer: ● Squamous cells: These are flat cells in the upper (outer) part of the epidermis, which are constantly shed as new ones form. When these cells grow out of control, they can develop into squamous cell skin cancer (also called squamous cell carcinoma). -

Pigment and Hair Disorders
Pigment and Hair Disorders Mohammed Al-Haddab,MD,FRCPC Assistant Professor, Consultant Dermatologist, Dermasurgeon Objectives • To be familiar with physiology of melanocytes and skin color. • To be familiar with common cutaneous pigment disorders, pathophysiology, clinical presentation and treatment • To be familiar with physiology of hair follicle • To be familiar with common hair disorders, both acquired and congenital, their presentation, investigation and management • Reference is the both the lecture and the TEXTBOOK Skin Pigment • Reduced hemoglobin: blue • Oxyhemoglobin: red • Carotenoids : yellow • Melanin : brown • Human skin color is classified according to Fitzpatrick skin phototype. www.steticsensediodolaser.es www.ijdvl.com Vitiligo • Incidence 1% • Early onset • A chronic autoimmune disease with genetic predisposition • Complete absence of melanocytes • Could affect skin, hair, retina, but Iris color no change • Rarely could be associated with: alopecia areata, thyroid disease, pernicious anemia, diabetes mellitus • Koebner phenomenon Vitiligo • Ivory white macules and patches with sharp convex margins • Slowly progressive or present abruptly then stabilize with time • Focal • Segmental • Generalized (commonest) • Trichrome • Acral • Poliosis www.metro.co.uk www.dermrounds.com www.medscape.com www.jaad.org Vitiligo • Diagnosis usually clinically • Wood’s lamp for early vitiligo, white person • Pathology shows normal skin with no melanocytes Differential Diagnosis of Vitiligo • Pityriasis alba • leprosy • Hypopigmented pityriasis -

Hair Follicle Tumors
Hair Follicle Tumors 803-808-7387 www.gracepets.com These notes are provided to help you understand the diagnosis or possible diagnosis of cancer in your pet. For general information on cancer in pets ask for our handout “What is Cancer”. Your veterinarian may suggest certain tests to help confirm or eliminate diagnosis, and to help assess treatment options and likely outcomes. Because individual situations and responses vary, and because cancers often behave unpredictably, science can only give us a guide. However, information and understanding for tumors in animals is improving all the time. We understand that this can be a very worrying time. We apologize for the need to use some technical language. If you have any questions please do not hesitate to ask us. What is this tumor? This is one of many similar tumors that arise by disordered growth of the hair follicles. These tumors are almost all benign and can be permanently cured by total surgical removal. Some occur at multiple sites within the same animal. This family of tumors grade into each other. Precise nomenclature is usually irrelevant as almost all are benign. What do we know about the cause? The reason why a particular pet may develop this, or any cancer, is not straightforward. Cancer is often seemingly the culmination of a series of circumstances that come together for the unfortunate individual. Cross Section of Skin & Hair Follicle B-catenin is required for differentiation of skin cells into hair follicles. If there is over-production of this chemical in the body, hair follicle tumors develop. -

Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe Between 2009 and 2018
microorganisms Article Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018 Anna Beltrame 1,* , Gianfranco Barabino 2, Yiran Wei 2, Andrea Clapasson 2, Pierantonio Orza 1, Francesca Perandin 1 , Chiara Piubelli 1 , Geraldo Badona Monteiro 1, Silvia Stefania Longoni 1, Paola Rodari 1 , Silvia Duranti 1, Ronaldo Silva 1 , Veronica Andrea Fittipaldo 3 and Zeno Bisoffi 1,4 1 Department of Infectious, Tropical Diseases and Microbiology, I.R.C.C.S. Sacro Cuore Don Calabria Hospital, Via Sempreboni 5, 37024 Negrar di Valpolicella, Italy; [email protected] (P.O.); [email protected] (F.P.); [email protected] (C.P.); [email protected] (G.B.M.); [email protected] (S.S.L.); [email protected] (P.R.); [email protected] (S.D.); [email protected] (R.S.); zeno.bisoffi@sacrocuore.it (Z.B.) 2 Dermatological Clinic, National Reference Center for Hansen’s Disease, Ospedale Policlinico San Martino, Sistema Sanitario Regione Liguria, Istituto di Ricovero e Cura a Carattere Scientifico per l’Oncologia, Largo Rosanna Benzi 10, 16132 Genoa, Italy; [email protected] (G.B.); [email protected] (Y.W.); [email protected] (A.C.) 3 Oncology Department, Mario Negri Institute for Pharmacological Research I.R.C.C.S., Via Giuseppe La Masa 19, 20156 Milano, Italy; vafi[email protected] 4 Department of Diagnostic and Public Health, University of Verona, P.le L. A. Scuro 10, 37134 Verona, Italy * Correspondence: [email protected]; Tel.: +39-045-601-4748 Received: 30 June 2020; Accepted: 23 July 2020; Published: 24 July 2020 Abstract: Leprosy is a chronic neglected infectious disease that affects over 200,000 people each year and causes disabilities in more than four million people in Asia, Africa, and Latin America. -

NAIL CHANGES in RECENT and OLD LEPROSY PATIENTS José M
NAIL CHANGES IN RECENT AND OLD LEPROSY PATIENTS José M. Ramos,1 Francisco Reyes,2 Isabel Belinchón3 1. Department of Internal Medicine, Hospital General Universitario de Alicante, Alicante, Spain; Associate Professor, Department of Medicine, Miguel Hernández University, Spain; Medical-coordinator, Gambo General Rural Hospital, Ethiopia 2. Medical Director, Gambo General Rural Hospital, Ethiopia 3. Department of Dermatology, Hospital General Universitario de Alicante, Alicante, Spain; Associate Professor, Department of Medicine, Miguel Hernández University, Spain Disclosure: No potential conflict of interest. Received: 27.09.13 Accepted: 21.10.13 Citation: EMJ Dermatol. 2013;1:44-52. ABSTRACT Nails are elements of skin that can often be omitted from the dermatological assessment of leprosy. However, there are common nail conditions that require special management. This article considers nail presentations in leprosy patients. General and specific conditions will be discussed. It also considers the common nail conditions seen in leprosy patients and provides a guide to diagnosis and management. Keywords: Leprosy, nails, neuropathy, multibacillary leprosy, paucibacillary leprosy, acro-osteolysis, bone atrophy, type 2 lepra reaction, anonychia, clofazimine, dapsone. INTRODUCTION Leprosy can cause damage to the nails, generally indirectly. There are few reviews about the Leprosy is a chronic granulomatous infection affectation of the nails due to leprosy. Nails are caused by Mycobacterium leprae, known keratin-based elements of the skin structure that since ancient times and with great historical are often omitted from the dermatological connotations.1 This infection is not fatal but affects assessment of leprosy. However, there are the skin and peripheral nerves. The disease causes common nail conditions that require diagnosis cutaneous lesions, skin lesions, and neuropathy, and management. -

Just a Cutaneous (Keratotic) Horn?
BMJ 2019;364:l595 doi: 10.1136/bmj.l595 (Published 7 March 2019) Page 1 of 2 Endgames BMJ: first published as 10.1136/bmj.l595 on 7 March 2019. Downloaded from ENDGAMES SPOT DIAGNOSIS Just a cutaneous (keratotic) horn? Jane Wilcock general practitioner, Yvonne Savage Silverdale Medical Practice, Salford, Manchester, UK A 70 year old woman attended a dermatologist with a lesion on In this case, the speed of growth made a keratoacanthoma a the dorsum of her right hand (fig 1). It had appeared over eight possibility. However, the patient also had several risk factors weeks and was painless but unsightly. She reported good health for squamous cell cancer: age, sun exposure, past lymphoma,1 and no history of warts. Fifteen years ago, she had lymphoma past chemotherapy, and no history of warts. Other invasive treated by chemotherapy; her last treatment (biological therapy) features of squamous cell cancer relevant to this case include had finished seven years ago and she had been well since. She the lesion’s arrival over eight weeks and its wide, thick, red base had holidayed in Australia for three months at a time over the with a diameter larger than the height of the horn. About 35% 2 last three years and more recently had driven frequently from of keratotic horns are invasive squamous cell cancers. http://www.bmj.com/ northern England to the south coast while a close relative was Invasive squamous cell cancer is a non-melanotic skin ill. She said she was careful to use sunscreen. malignancy with a good prognosis but may metastasise to the lymph nodes. -

UC Davis Dermatology Online Journal
UC Davis Dermatology Online Journal Title Alopecia areata with white hair regrowth: case report and review of poliosis Permalink https://escholarship.org/uc/item/1xk5b26v Journal Dermatology Online Journal, 20(9) Authors Jalalat, Sheila Z Kelsoe, John R Cohen, Philip R Publication Date 2014 DOI 10.5070/D3209023902 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 20 Number 9 September 2014 Case Presentation Alopecia areata with white hair regrowth: case report and review of poliosis Sheila Z. Jalalat BS1, John R. Kelsoe MD2, and Philip R. Cohen MD3 Dermatology Online Journal 20 (9): 8 1Medical School, The University of Texas Medical Branch, Galveston, Texas 2Department of Psychiatry, University of San Diego, San Diego, California 3Division of Dermatology, University of San Diego, San Diego, California Correspondence: Sheila Z. Jalalat, BS Philip R. Cohen, MD 6207 Retlin Ct. 10991 Twinleaf Court Houston, TX 77041 San Diego, California 92131 Email: [email protected] Email: [email protected] Abstract Alopecia areata is thought to be a T-cell mediated and cytokine mediated autoimmune disease that results in non-scarring hair loss. Poliosis has been described as a localized depigmentation of hair caused by a deficiency of melanin in hair follicles. A 57- year-old man with a history of alopecia areata developed white hair regrowth in areas of previous hair loss. We retrospectively reviewed the medical literature using PubMed, searching: (1) alopecia areata and (2) poliosis. Poliosis may be associated with autoimmune diseases including alopecia areata, as described in our case.