Conversion of Perovskite to Anatase and Tio2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

19660017397.Pdf
.. & METEORITIC RUTILE Peter R. Buseck Departments of Geology and Chemistry Arizona State University Tempe, Arizona Klaus Keil Space Sciences Division National Aeronautics and Space Administration Ames Research Center Mof fett Field, California r ABSTRACT Rutile has not been widely recognized as a meteoritic constituent. show, Recent microscopic and electron microprobe studies however, that Ti02 . is a reasonably widespread phase, albeit in minor amounts. X-ray diffraction studies confirm the Ti02 to be rutile. It was observed in the following meteorites - Allegan, Bondoc, Estherville, Farmington, and Vaca Muerta, The rutile is associated primarily with ilmenite and chromite, in some cases as exsolution lamellae. Accepted for publication by American Mineralogist . Rutile, as a meteoritic phase, is not widely known. In their sunanary . of meteorite mineralogy neither Mason (1962) nor Ramdohr (1963) report rutile as a mineral occurring in meteorites, although Ramdohr did describe a similar phase from the Faxmington meteorite in his list of "unidentified minerals," He suggested (correctly) that his "mineral D" dght be rutile. He also ob- served it in several mesosiderites. The mineral was recently mentioned to occur in Vaca Huerta (Fleischer, et al., 1965) and in Odessa (El Goresy, 1965). We have found rutile in the meteorites Allegan, Bondoc, Estherville, Farming- ton, and Vaca Muerta; although nowhere an abundant phase, it appears to be rather widespread. Of the several meteorites in which it was observed, rutile is the most abundant in the Farmington L-group chondrite. There it occurs in fine lamellae in ilmenite. The ilmenite is only sparsely distributed within the . meteorite although wherever it does occur it is in moderately large clusters - up to 0.5 mn in diameter - and it then is usually associated with chromite as well as rutile (Buseck, et al., 1965), Optically, the rutile has a faintly bluish tinge when viewed in reflected, plane-polarized light with immersion objectives. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine
nanomaterials Review Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine Daniel Ziental 1 , Beata Czarczynska-Goslinska 2, Dariusz T. Mlynarczyk 3 , Arleta Glowacka-Sobotta 4, Beata Stanisz 5, Tomasz Goslinski 3,* and Lukasz Sobotta 1,* 1 Department of Inorganic and Analytical Chemistry, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] 2 Department of Pharmaceutical Technology, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] 3 Department of Chemical Technology of Drugs, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] 4 Department and Clinic of Maxillofacial Orthopedics and Orthodontics, Poznan University of Medical Sciences, Bukowska 70, 60-812 Poznan, Poland; [email protected] 5 Department of Pharmaceutical Chemistry, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] * Correspondence: [email protected] (T.G.); [email protected] (L.S.) Received: 4 January 2020; Accepted: 19 February 2020; Published: 23 February 2020 Abstract: Metallic and metal oxide nanoparticles (NPs), including titanium dioxide NPs, among polymeric NPs, liposomes, micelles, quantum dots, dendrimers, or fullerenes, are becoming more and more important due to their potential use in novel medical therapies. Titanium dioxide (titanium(IV) oxide, titania, TiO2) is an inorganic compound that owes its recent rise in scientific interest to photoactivity. After the illumination in aqueous media with UV light, TiO2 produces an array of reactive oxygen species (ROS). The capability to produce ROS and thus induce cell death has found application in the photodynamic therapy (PDT) for the treatment of a wide range of maladies, from psoriasis to cancer. -

Electronic Structure and Optical Properties of Prominent Phases of Tio2: First-Principles Study
Pramana – J. Phys. (2017) 89:5 © Indian Academy of Sciences DOI 10.1007/s12043-017-1400-5 Electronic structure and optical properties of prominent phases of TiO2: First-principles study SANTOSH SINGH and MADHVENDRA NATH TRIPATHI∗ Department of Pure and Applied Physics, Guru Ghasidas Vishwavidyalaya (Central University), Koni, Bilaspur 495 009, India ∗Corresponding author. E-mail: [email protected] Published online 19 June 2017 Abstract. First-principles study based on density functional theory (DFT) of two prominent phases, the rutile and the anatase phases, of titanium dioxide (TiO2) are reported within the generalized gradient approximation (GGA). Our calculated band structure shows that there is a significant presence of O-2p and Ti-3d hybridization in the valence bands. These bands are well separated from the conduction bands by a direct band gap value of 1.73 eV in the rutile phase and an indirect band gap value of 2.03 eV in the anatase phase, from to X. Our calculations reproduced the peaks in the conduction and valence band, are in good agreement with experimental observations. Our structural optimization for the rutile and anatase phase led to lattice parameter values of 4.62 Å and 2.99 Å rutile and 3.80 Å and 9.55 Å for anatase for a and c. The static dielectric values 7.0 and 5.1 for the rutile and anatase phases respectively are in excellent agreement with experimental results. Our calculation of optical properties reveals that maximum value of the transmittance in anatase phase of TiO2 may be achieved by considering the anisotropic behaviour of the optical spectra in the optical region for transparent conducting application. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Combustion Synthesis, Sintering, Characterization, and Properties
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 9639016, 9 pages https://doi.org/10.1155/2019/9639016 Research Article Calcium Titanate from Food Waste: Combustion Synthesis, Sintering, Characterization, and Properties Siriluk Cherdchom,1 Thitiwat Rattanaphan,1 and Tawat Chanadee 1,2 1Department of Materials Science and Technology, Faculty of Science, Prince of Songkla University (PSU), Hat Yai, Songkhla 90110, &ailand 2Ceramic and Composite Materials Engineering Research Group (CMERG), Center of Excellence in Materials Engineering (CEME), Prince of Songkla University (PSU), Hat Yai, Songkhla 90110, &ailand Correspondence should be addressed to Tawat Chanadee; [email protected] Received 31 August 2018; Revised 20 December 2018; Accepted 3 January 2019; Published 12 February 2019 Academic Editor: Andres Sotelo Copyright © 2019 Siriluk Cherdchom et al. )is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3) was combustion synthesized from a calcium source of waste duck eggshell, anatase titanium dioxide (A-TiO2), and magnesium (Mg). )e eggshell and A-TiO2 were milled for 30 min in either a high-energy planetary mill or a conventional ball mill. )ese powders were then separately mixed with Mg in a ball mill. After synthesis, the combustion products were leached and then sintered to produce CaTiO3 ceramic. Analytical characterization of the as-leached combustion products revealed that the product of the combustion synthesis of duck eggshell + A-TiO2 that had been high-energy-milled for 30 min before synthesis comprised a single perovskite phase of CaTiO3. -

CRYSTAL SRUKTUR of CALCIUM TITANATE (Catio3) PHOSPHOR DOPED with PRASEODYMIUM and ALUMINIUM IONS
CRYSTAL SRUKTUR OF CALCIUM TITANATE (CaTiO3) PHOSPHOR DOPED WITH PRASEODYMIUM AND ALUMINIUM IONS STRUKTUR KRISTAL FOSFOR KALSIUM TITANIA DIDOPKAN DENGAN ION PRASEODYMIUM DAN ALUMINIUM IONS Siti Aishah Ahmad Fuzi1* and Rosli Hussin2 1 Material Technology Group, Industrial Technology Division, Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor Darul Ehsan, Malaysia. 2 Department of Physics, Faculty of Science, Universiti Teknologi Malaysia, 81310 Skudai, Johor 1*[email protected], [email protected] Abstract The past three decades have witnessed rapid growth in research and development of luminescence phenomenon because of their diversity in applications. In this paper, Calcium Titanate (CaTiO3) was studied to find a new host material with desirable structural properties for luminescence-based applications. Solid state reactions o 3+ methods were used to synthesis CaTiO3 at 1000 C for 6 hours. Crystal structure of CaTiO3 co-doped with Pr 3+ and Al were investigated using X-Ray Diffraction (XRD) method. Optimum percentage to synthesis CaTiO3 was 3+ obtained at 40 mol%CaO-60 mol%TiO2 with a single doping of 1 mol%Pr . However, a crystal structure of 4 mol% of Al3+ co-doped with Pr3+ was determined as an optimum parameter which suitable for display imaging. Keywords: calcium titanate, anatase, rutile Abstrak Semenjak tiga dekad yang lalu telah menunjukkan peningkatan yang ketara bagi kajian dan pembangunan dalam bidang fotolumiscen. Peningkatan ini berkembang dengan meluas disebabkan oleh kebolehannya untuk diaplikasikan dalam pelbagai kegunaan harian. Dalam manuskrip ini, kalsium titania (CaTiO3) telah dikaji untuk mencari bahan perumah dengan sifat struktur yang bersesuaian bagi aplikasi luminescen. Tindak balas keadaan o pepejal telah digunakan bagi mensintesis CaTiO3 pada suhu 1000 C selama 6 jam. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
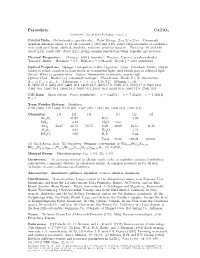
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy.