Neurophysiological Aspects of the Trigeminal Sensory System: an Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Mandibular Nerve - Vc Or VIII by Prof
The Mandibular Nerve - Vc or VIII by Prof. Dr. Imran Qureshi The Mandibular nerve is the third and largest division of the trigeminal nerve. It is a mixed nerve. Its sensory root emerges from the posterior region of the semilunar ganglion and is joined by the motor root of the trigeminal nerve. These two nerve bundles leave the cranial cavity through the foramen ovale and unite immediately to form the trunk of the mixed mandibular nerve that passes into the infratemporal fossa. Here, it runs anterior to the middle meningeal artery and is sandwiched between the superior head of the lateral pterygoid and tensor veli palatini muscles. After a short course during which a meningeal branch to the dura mater, and the nerve to part of the medial pterygoid muscle (and the tensor tympani and tensor veli palatini muscles) are given off, the mandibular trunk divides into a smaller anterior and a larger posterior division. The anterior division receives most of the fibres from the motor root and distributes them to the other muscles of mastication i.e. the lateral pterygoid, medial pterygoid, temporalis and masseter muscles. The nerve to masseter and two deep temporal nerves (anterior and posterior) pass laterally above the medial pterygoid. The nerve to the masseter continues outward through the mandibular notch, while the deep temporal nerves turn upward deep to temporalis for its supply. The sensory fibres that it receives are distributed as the buccal nerve. The 1 | P a g e buccal nerve passes between the medial and lateral pterygoids and passes downward and forward to emerge from under cover of the masseter with the buccal artery. -

Morphometry and Morphology of Foramen Petrosum in Indian Population
Basic Sciences of Medicine 2020, 9(1): 8-9 DOI: 10.5923/j.medicine.20200901.02 Morphometry and Morphology of Foramen Petrosum in Indian Population Rajani Singh1,*, Nand Kishore Gupta1, Raj Kumar2 1Department of Anatomy, Uttar Pradesh University of Medical Sciences Saifai 206130 Etawah UP India 2Department of Neurosugery Uttar Pradesh University of Medical Sciences Saifai 206130 Etawah UP India Abstract Greater wing of sphenoid contains three constant foramina, Foramen ovale, foramen rotundum and foramen spinosum. The presence of foramen Vesalius and foramen petrosum are inconsistent. Normally foramen ovale transmits mandibular nerve, accessory meningeal artery, lesser petrosal nerve and emissary vein. When foramen petrosum is present, lesser petrosal nerve passes through petrosal foramen instead of foramen ovale. Lesser petrosal nerve distribute postganglionic fibers from otic ganglion to parotid gland. In absence of knowledge of petrosal foramen transmitting lesser petrosal nerve, the clinician may damage the nerve during skull base surgery creating complications like hyperemia of face and profuse salivation from the parotid gland (following atropine administration), lacrimation (crocodile tears syndrome) and mucus nasal secretion. Considering clinical implications associated with petrosal foramen, the study was carried out. The aim of the study is to determine the prevalence of petrosal foramen in Indian Population and to bring out associated clinical significance. The study was conducted in the department of Anatomy UPUMS Saifai Etawah Indian. 30 half skulls were observed for the presence of petrosal foramina and morphometry was also done. Literature search was carried out, our findings were compared with previous work and associated clinical implications were bought out. Keywords Petrosal foramen, Lesser petrosal nerve, Foramen ovale patients. -

Clinical Anatomy of the Trigeminal Nerve
Clinical Anatomy of Trigeminal through the superior orbital fissure Nerve and courses within the lateral wall of the cavernous sinus on its way The trigeminal nerve is the fifth of to the trigeminal ganglion. the twelve cranial nerves. Often Ophthalmic Nerve is formed by the referred to as "the great sensory union of the frontal nerve, nerve of the head and neck", it is nasociliary nerve, and lacrimal named for its three major sensory nerve. Branches of the ophthalmic branches. The ophthalmic nerve nerve convey sensory information (V1), maxillary nerve (V2), and from the skin of the forehead, mandibular nerve (V3) are literally upper eyelids, and lateral aspects "three twins" carrying information of the nose. about light touch, temperature, • The maxillary nerve (V2) pain, and proprioception from the enters the middle cranial fossa face and scalp to the brainstem. through foramen rotundum and may or may not pass through the • The three branches converge on cavernous sinus en route to the the trigeminal ganglion (also called trigeminal ganglion. Branches of the semilunar ganglion or the maxillary nerve convey sensory gasserian ganglion), which contains information from the lower eyelids, the cell bodies of incoming sensory zygomae, and upper lip. It is nerve fibers. The trigeminal formed by the union of the ganglion is analogous to the dorsal zygomatic nerve and infraorbital root ganglia of the spinal cord, nerve. which contain the cell bodies of • The mandibular nerve (V3) incoming sensory fibers from the enters the middle cranial fossa rest of the body. through foramen ovale, coursing • From the trigeminal ganglion, a directly into the trigeminal single large sensory root enters the ganglion. -

A Review of the Mandibular and Maxillary Nerve Supplies and Their Clinical Relevance
AOB-2674; No. of Pages 12 a r c h i v e s o f o r a l b i o l o g y x x x ( 2 0 1 1 ) x x x – x x x Available online at www.sciencedirect.com journal homepage: http://www.elsevier.com/locate/aob Review A review of the mandibular and maxillary nerve supplies and their clinical relevance L.F. Rodella *, B. Buffoli, M. Labanca, R. Rezzani Division of Human Anatomy, Department of Biomedical Sciences and Biotechnologies, University of Brescia, V.le Europa 11, 25123 Brescia, Italy a r t i c l e i n f o a b s t r a c t Article history: Mandibular and maxillary nerve supplies are described in most anatomy textbooks. Accepted 20 September 2011 Nevertheless, several anatomical variations can be found and some of them are clinically relevant. Keywords: Several studies have described the anatomical variations of the branching pattern of the trigeminal nerve in great detail. The aim of this review is to collect data from the literature Mandibular nerve and gives a detailed description of the innervation of the mandible and maxilla. Maxillary nerve We carried out a search of studies published in PubMed up to 2011, including clinical, Anatomical variations anatomical and radiological studies. This paper gives an overview of the main anatomical variations of the maxillary and mandibular nerve supplies, describing the anatomical variations that should be considered by the clinicians to understand pathological situations better and to avoid complications associated with anaesthesia and surgical procedures. # 2011 Elsevier Ltd. -

05 Trigeminal System 2013.Pdf
Dental Neuroanatomy Thursday February 7th, 2013 David A. Morton, Ph.D. 5. THE TRIGEMINAL SYSTEM Somatic Sensation of the Face and Head Objectives 1. Outline the two pathways for facial sensation from the head. 2. Contrast facial sensation from the head and somatic sensation from the body. In what ways are they similar? Different? Try drawing this on the Haines atlas diagram at the end of the lecture. 3. Diagram the corneal reflex: the afferent and efferent limbs as well as nuclei involved in the brainstem. 4. If a person does not blink, how would you determine if the problem were in the sensory (afferent) limb, motor (efferent) limb, or brainstem interconnections for the corneal reflex? 5. Explain how a single, small medullary vascular lesion could abolish pain and temperature from the face on the right side and pain and temperature from the body on the left side. What vessel is most likely occluded? Introduction – The trigeminal system for the face and oral cavity is organized in a manner similar to the spinal cord. It has the equivalent of both the DCML pathway and the ALS pathway. The two trigeminal pathways will converge in the thalamus. The most confusing thing is that one of them descends before crossing and the other crosses immediately. Peripheral Receptors and Sensation Structures served by trigeminal system. 1. Cornea 2. Mucocutaneous tissues around mouth and nostrils. 3. Oral and nasal mucosae 4. Paranasal sinuses 5. Tongue (anterior two thirds) 6. Teeth and gums 7. Dura of anterior and middle cranial fossae 8. Skin of face to the vertex except angle of jaw 9. -

The Mandibular Nerve: the Anatomy of Nerve Injury and Entrapment
5 The Mandibular Nerve: The Anatomy of Nerve Injury and Entrapment M. Piagkou1, T. Demesticha2, G. Piagkos3, Chrysanthou Ioannis4, P. Skandalakis5 and E.O. Johnson6 1,3,4,5,6Department of Anatomy, 2Department of Anesthesiology, Metropolitan Hospital Medical School, University of Athens Greece 1. Introduction The trigeminal nerve (TN) is a mixed cranial nerve that consists primarily of sensory neurons. It exists the brain on the lateral surface of the pons, entering the trigeminal ganglion (TGG) after a few millimeters, followed by an extensive series of divisions. Of the three major branches that emerge from the TGG, the mandibular nerve (MN) comprises the 3rd and largest of the three divisions. The MN also has an additional motor component, which may run in a separate facial compartment. Thus, unlike the other two TN divisions, which convey afferent fibers, the MN also contains motor or efferent fibers to innervate the muscles that are attached to mandible (muscles of mastication, the mylohyoid, the anterior belly of the digastric muscle, the tensor veli palatini, and tensor tympani muscle). Most of these fibers travel directly to their target tissues. Sensory axons innervate skin on the lateral side of the head, tongue, and mucosal wall of the oral cavity. Some sensory axons enter the mandible to innervate the teeth and emerge from the mental foramen to innervate the skin of the lower jaw. An entrapment neuropathy is a nerve lesion caused by pressure or mechanical irritation from some anatomic structures next to the nerve. This occurs frequently where the nerve passes through a fibro-osseous canal, or because of impingement by an anatomic structure (bone, muscle or a fibrous band), or because of the combined influences on the nerve entrapment between soft and hard tissues. -

Maxillary Nerve Variations and Its Clinical Significance
Sai Pavithra .R et al /J. Pharm. Sci. & Res. Vol. 6(4), 2014, 203-205 Maxillary Nerve Variations and Its Clinical Significance Sai Pavithra .R,Thenmozhi M.S Department of Anatomy, Saveetha Dental College Tamil nadu. Abstract: The aim of this review is to collect data from the literature and gives a detailed description of the innervation of the maxilla. We carried out a search of studies published in PubMed up to 2011, including clinical and anatomical studies .several articles has has been published regarding the anatomical variations of the maxillary nerve supplies. The purpose of this paper is to demonstrate the variation in the paths and communications of the maxillary nerve which should be considered by the clinicians as nerve supply,assumed prime importance to oral surgeons to understand better and to avoid complications associated with anaesthesia and surgical procedures. Key words: maxillary nerve, clinical variations THE MAXILLARY NERVE: foramen is usually a single foramen but several studies 1. Anatomical variations of the maxillary nerve supply. have proven to have two or three foramen[2-8]. A low 2. Anatomical variations of the infraorbital nerve. percentage (4.7%) was observed during a study on 3. Anatomical variations of the superior alveolar nerve 1064 skulls, with a higher frequency on the left side, 4.Anatomical variations of the greaterpalatine nerve. both in male and in female skulls[9]. The distance from 5. Anatomical variations of the nasopalatine nerve. the infraorbital foramen to the inferior border of the [10-13] orbital rim is from 4.6 to 10.4 mm . AXILLARY NERVE: Since the infraorbital nerve block is often used to The maxillary nerve is a sensory nerve. -

Intracranial Stimulation of the Trigeminal Nerve in Man II
Journal of Neurology, Neurosurgery, and Psychiatry 1986;49:419-427 Intracranial stimulation of the trigeminal nerve in man II. Reflex responses GIORGIO CRUCCU, DAVID BOWSHER From the Pain ReliefFoundation and Department ofNeurosciences, Walton Hospital, Liverpool, UK SUMMARY The reflex responses evoked by direct electrical stimulation of the intracranial portion of the trigeminal nerve have been studied in 16 subjects undergoing percutaneous retrogasserian ther- mocoagulation for the treatment of trigeminal neuralgia affecting the second or third division. In the obicularis oculi muscle, early and late responses similar to the RI and R2 components of the blink reflex were recorded. The former could be evoked only by stimulation of the second division and its latency was consistent with intermediately fast afferents. A late reflex (50-70 ms) was occa- sionally recorded from the anterior belly of the digastric muscle. The response was sometimes followed by a later activity and showed the features of a polysynaptic reflex. No response was obtained in the jaw elevators when fully relaxed. With the subject voluntarily clenching his teeth, both an early "H-like" response and two silent periods in the background EMG were obtained. The second silent period was similar in the muscles ipsi- and contralateral to intracranial stimulation, while the first silent period was longer in the ipsilateral muscles. Possible mechanisms contributing to the inhibition following stimulation of the mixed portion of the nerve are discussed. Owing to difficulties -
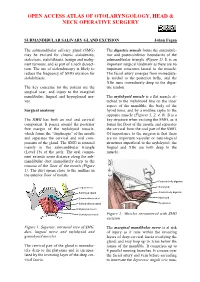
Submandibular Gland Excision
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY SUBMANDIBULAR SALIVARY GLAND EXCISION Johan Fagan The submandibular salivary gland (SMG) The digastric muscle forms the anteroinfe- may be excised for chronic sialadenitis, rior and posteroinferior boundaries of the sialectasis, sialolithiasis, benign and malig- submandibular triangle (Figure 2). It is an nant tumours, and as part of a neck dissect- important surgical landmark as there are no tion. The use of sialendoscopy is likely to important structures lateral to the muscle. reduce the frequency of SMG excision for The facial artery emerges from immediate- sialolithiasis. ly medial to the posterior belly, and the XIIn runs immediately deep to the digas- The key concerns for the patient are the tric tendon. surgical scar, and injury to the marginal mandibular, lingual and hypoglossal ner- The mylohyoid muscle is a flat muscle at- ves. tached to the mylohyoid line on the inner aspect of the mandible, the body of the Surgical anatomy hyoid bone, and by a midline raphe to the opposite muscle (Figures 1, 2, 4, 8). It is a The SMG has both an oral and cervical key structure when excising the SMG, as it component. It passes around the posterior forms the floor of the mouth, and separates free margin of the mylohyoid muscle, the cervical from the oral part of the SMG. which forms the “diaphragm” of the mouth Of importance to the surgeon is that there and separates the cervical and oral com- are no important vascular or neurological ponents of the gland. The SMG is situated structures superficial to the mylohyoid; the mainly in the submandibular triangle lingual and XIIn are both deep to the (Level 1b) of the neck. -

I. Otic Ganglion
ACTA THERIOLOGICA Vol. 33, 10: 115— 120, 1988 Parasympathetic Ganglia in the Head of Western Hedgehog (Erinaceus europaeus). I. Otic ganglion Jan GIENC, Tadeusz KUDER & Aleksander SZCZURKOWSKI Gienc J., Kuder T. & Szczurkowski A., 1988: Parasympathetic ganglia in the head of western hedgehog(Erinaceus europaeus). I. Otic ganglion. Acta theriol., 33, 10: 115— 120. [With Plates V—VI] Using the thiocholine method of Koelle and Friedenwald and histo logical techniques the otic ganglion of the western hedgehog,Erinaceus europaeus Linnaeus, 1758 from the Province of Wroclaw was studied. The ganglion was found to be a single oval cluster of neurons of gan glionic type, 2.4 mm long and 0.9 mm broad. The otic ganglion is situated at the medial side of the mandibular nerve closely below the maxillary artery. The ganglion is composed of typical ganglionic neurons in compact arrangement, and is surrounded by a thick connective-tissue capsule. [Department of Anatomy, Institute of Biology, Pedagogical College, Rew. Październikowej Str. 33, 25-518 Kielce, Poland). 1. INTRODUCTION The parasympathetic cephalic ganglia have been studied in many species of birds and mammals, and among the latter the rodents have been given most attention (Gienc & Kuder, 1980; Kuder, 1980, 1983a, 1983b, 1985). On the other hand, no similar data are available on the Insectivora. Earlier studies suggested certain correlations between the morphological features and the topography of the parasympathetic ganglia and the taxonomic position of animal species. In view of this, a study was untertaken on the cephalic parasympathetic ganglia in the hedgehog, since this might be of interest from the stand point of phylogenetic development. -

Trigeminal Nerve Trigeminal Neuralgia
Trigeminal nerve trigeminal neuralgia Dr. Gábor GERBER EM II Trigeminal nerve Largest cranial nerve Sensory innervation: face, oral and nasal cavity, paranasal sinuses, orbit, dura mater, TMJ Motor innervation: muscles of first pharyngeal arch Nuclei of the trigeminal nerve diencephalon mesencephalic nucleus proprioceptive mesencephalon principal (pontine) sensory nucleus epicritic motor nucleus of V. nerve pons special visceromotor or branchialmotor medulla oblongata nucleus of spinal trigeminal tract protopathic Segments of trigeminal nereve brainstem, cisternal (pontocerebellar), Meckel´s cave, (Gasserian or semilunar ganglion) cavernous sinus, skull base peripheral branches Somatotopic organisation Sölder lines Trigeminal ganglion Mesencephalic nucleus: pseudounipolar neurons Kovách Motor root (Radix motoria) Sensory root (Radix sensoria) Ophthalmic nerve (V/1) General sensory innervation: skin of the scalp and frontal region, part of nasal cavity, and paranasal sinuses, eye, dura mater (anterior and tentorial region) lacrimal gland Branches of ophthalmic nerve (V/1) tentorial branch • frontal nerve (superior orbital fissure outside the tendinous ring) o supraorbital nerve (supraorbital notch) o supratrochlear nerve (supratrochlear notch) o lacrimal nerve (superior orbital fissure outside the tendinous ring) o Communicating branch to zygomatic nerve • nasociliary nerve (superior orbital fissure though the tendinous ring) o anterior ethmoidal nerve (anterior ethmoidal foramen then the cribriform plate) (ant. meningeal, ant. nasal, -

Facial Nerve Marginal Mandibular Branch Lesion a Complication of Carotid Surgery
Published Ahead of Print on April 21, 2020 as 10.1212/WNL.0000000000009415 RESIDENT & FELLOW SECTION Pearls & Oy-sters: Facial nerve marginal mandibular branch lesion A complication of carotid surgery Kimberley Fleuren, MD, and Julie Staals, MD, PhD Correspondence Dr. Fleuren Neurology 2020;94:1-e3. doi:10.1212/WNL.0000000000009415 ® [email protected] Pearls c Iatrogenic injury of the marginal mandibular branch of the facial nerve is a rare complication of carotid endarterectomy and causes paresis of the ipsilateral lower lip. c The weakness may be detected by a full denture-type smile or by opening the mouth wide, showing that a person cannot expose his or her lower teeth on the paretic side. c Paresis of the marginal mandibular branch has minor to no clinical consequences but can have cosmetic consequences. Oy-sters c A marginal mandibular branch lesion may give the false appearance of a central facial nerve palsy. c Misinterpretation leads to overtesting and incorrect therapeutic strategy. Case 1 A 78-year-old woman underwent a left-sided carotid endarterectomy (CEA) because of significant carotid stenosis of 90%, after having had a minor ischemic stroke in the left hemisphere, from which she recovered completely.Beforesurgery,therewasnofacial asymmetry. On day 1 after surgery, the vascular surgeon contacted the neurologist for an urgent consult. The surgeon believed the patient had a left-sided facial paresis with a drooped mouth corner, fearing a new ischemic event in the contralateral, nonoperated right hemi- sphere. On neurologic examination, the resident in neurology noticed facial asymmetry (figure). The patient could not move her left lower lip; movements of the left upper lip and the other facial muscles were intact.