An Efficient Synthesis and Bio Efficacy Confirmation of Female Sex
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Efficacy of Azadirachta Indica and Eucalyptus Globulus Against Corcyra Cephalonica
Int. J. Adv. Res. Biol. Sci. (2019). 6(7): 119-123 International Journal of Advanced Research in Biological Sciences ISSN: 2348-8069 www.ijarbs.com DOI: 10.22192/ijarbs Coden: IJARQG(USA) Volume 6, Issue 7 -2019 Research Article DOI: http://dx.doi.org/10.22192/ijarbs.2019.06.07.015 Efficacy of Azadirachta indica and Eucalyptus globulus against Corcyra cephalonica Harminder Singh, Anita Singh* and Jora Singh Brar Department of Entomology, University College of Agriculture, Guru Kashi University, Talwandi Sabo, Bathinda Punjab-151302 Email ID*: [email protected] Abstract The four different concentrations (0.5, 1.0, 1.5 and 2.0 g) of Azadirachta indica and Eucalyptus globules powdered leaves were tested against all stages of rice-moth larvae. The result revealed that maximum mortality were observed at higher concentration (2.0 g) with both botanicals in first and second instar of Corcyra cephalonica. When compared both botanicals, E. globulus shows more effectiveness as compared to Azadirachta indica on larvae of rice moth. Therefore on small scale these plant powders having the insecticidal effect could be used as good alternatives for synthetic pesticides against this pest. Keywords: Corcyra cephalonica, Eucalyptus globulus, Azadirachta indica, Mortality Introduction C. cephalonica causes serious damage to stored grain by feeding, leaving dense and tough silken threads Corcyra cephalonica (Stainton) (Lepidoptera: (Ayyar, 1934; Prevett, 1964). The damage also form Pyralidae) is one of the most widely distributed critical frass which make grains useless for human insect pests of the stored grains (Osmen, 1984). It is consumption (Frenemore and Prakash, 1992). To commonly known as Rice moth. -

Stored Grain Insects and Pea Weevil (Live) Insects Large – Dead Or Alive
To whom it may concern, Proposal for GTA Standards change regarding Cereal grains for categories: Stored Grain Insects and Pea Weevil (live) Insects Large – dead or alive Currently there is a lack of reference with insects of NIL tolerance applied by DA for export and that listed within GTA standards. This has the potential to cause contract disputes especially in the grower direct to port transactions. At present if a supplier delivers grain with live insects for example Small-eyed flour beetles and Black fungus beetles, there is no reference in the standards that declare such insects as NIL tolerance. If the buyer was loading a container direct for export this would pose a problem due to the NIL tolerance being applied by DA for export phytosanitary requirements. These insects are in the same category as Psocids which are listed in GTA receival standards. I would like to see the GTA "Stored Grain Insects and Pea Weevil (live)" & "Insects Large – dead or alive" reflect the Department of Agriculture PEOM 6a: Pests, Diseases and Contaminants of Grain and Plant Products (excluding horticulture) http://www.agriculture.gov.au/SiteCollectionDocuments/aqis/exporting/plants-exports-operation-manual/vol6A.pdf I put forward the motion to have all major and minor injurious pests listed within PEOM 6a that apply to cereal grains to be of NIL tolerance within the GTA standards. 1) This would involve moving the Hairy Fungus Beetle Typhaea stercorea from “Insects Large – dead or alive” to the list of “Stored Grain Insects and Pea Weevil (live)”. Thus taking it from a tolerance level of 3 per half litre to NIL. -

Effect of Various Cereals on the Development of Corcyra Cephalonica (Stainton ) and Its Egg Parasitoid Trichogramma Chilonis (Ishii) M
ISSN 0258-7122 (Print), 2408-8293 (Online) Bangladesh J. Agril. Res. 41(1): 183-194, March 2016 EFFECT OF VARIOUS CEREALS ON THE DEVELOPMENT OF CORCYRA CEPHALONICA (STAINTON ) AND ITS EGG PARASITOID TRICHOGRAMMA CHILONIS (ISHII) M. NASRIN1, M. Z. ALAM2, S. N. ALAM3 M. R. U. MIAH4 AND M. M. HOSSAIN5 Abstract Eight types of cereals viz., wheat grain, chopped wheat, paddy grain, rice grain, maize grain, chopped maize, rice bran, mixture of rice bran and chopped rice were fed to observe the development parameters like egg, larva, pupa and adult stages of Corcyra cephalonica (stainton) for three consecutive generations. The parasitism efficiency of Trichogramma chilonis (Ishii) was also evaluated on the resultant host eggs of C. cephalonica. The C. cephalonica revealed the highest number of eggs (115.6 female1), higher hatchability (92.9%), extented larval duration (45.9 days), increased larval weight (0.058 gm), survival rate (88.3%), adult emergence rate (93.5%), and male and female longevity (7.7, 7.2 days respectively) when they were reared on chopped wheat. On the other hand, the lowest number of egg was found on paddy husk (29.2 female-1). The lowest hatchability (45.6%), larval duration (45.9 days), larval weight (0.029gm), and survival rate (38.2%), pupal duration (17.9 days) adult emergence (42.0%), male and female longevity (4.8 and 4.7 days respectively) were found on paddy husk. The effect of food materials also reflected on the parasitism efficiency of the egg parasitoid T. chilonis. The highest percent egg parasitization was done by the T. -

Biodiversity and Faunistic Studies of the Family Pyralidae
Pak. j. life soc. Sci. (2017), 15(2): 126-132 E-ISSN: 2221-7630;P-ISSN: 1727-4915 Pakistan Journal of Life and Social Sciences www.pjlss.edu.pk RESEARCH ARTICLE Biodiversity and Faunistic Studies of the Family Pyralidae (Lepidoptera) from Pothwar Region, Punjab, Pakistan Tazeel Ahmad 1, Zahid Mahmood Sarwar 2*, Mamoona Ijaz 2 , Muhammad Sajjad and Muhammad Bin yameen 2 1 Pir Mehr Ali Shah -Arid Agriculture University Rawalpindi 2 Department of Entomology, Bahauddin Zakariya University, Multan 60800, Pakistan ARTICLE INFO ABSTRACT Received: Jan 24, 2017 Snout moths (Lepidoptera: Pyralidae) are economic pests of agricultural crops and Accepted: Aug 18, 2017 forest plantations. To explore new species via taxonomic identification, 127 specimens of moths were collected from different areas of Pothwar region of Keywords Pakistan including Chakwal, Attock, Jhelum and Rawalpindi districts during 2009- Pothwar 2010. The characters of the specimens were identified at species level by using Pyralidae Hampson’s key and other taxonomic resources. All of these species new to Pyralid Snout Moths moth fauna of Pothwar region namely: Galleria mellonella Linnaeus., Achroia Taxonomic keys grisella Fabricius., Corcyra cephalonica Staint., Chilo simplex Butler., Scirpophaga auriflua Zeller., S. chrysorrhoa Zeller., Leucinodes orbonalis Guenee., Zinckenia fascialis Cramer and Cnaphalocrocis medinalis Guenee. are reported for the first time from Pothwar region. To understand the biodiversity of moths, distribution of the species were also studied for each district of Pothwar region. The distribution pattern of Corcyra cephalonica (42), Leucinodes orbonalis (18), Scirpophaga auriflua (10) and Scirpophaga chrysorrhoa (9), Chilo simplex (6), Zinckenia fascialis (7), Cnaphalocrocis medinalis (10), Galleria mellonella (24) and Achroia *Corresponding Author: grisella (1) was observed. -

Toxicity, Antifeedant, Egg Hatchability and Adult Emergence Effect of Piper Nigrum L. and Jatropha Curcas L. Extracts Against Ri
Vol. 7(18), pp. 1255-1262, 10 May, 2013 DOI: 10.5897/JMPR12.600 Journal of Medicinal Plants Research ISSN 1996-0875 ©2013 Academic Journals http://www.academicjournals.org/JMPR Full Length Research Paper Toxicity, antifeedant, egg hatchability and adult emergence effect of Piper nigrum L. and Jatropha curcas L. extracts against rice moth, Corcyra cephalonica (Stainton) Mousa Khani 1,2 , Rita Muhamad Awang 2*, Dzolkhifli Omar 2 and Mawardi Rahmani 3 1Cultivation and Development Department of Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran. 2Department of Plant Protection, Faculty of Agriculture, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. 3Department of Chemistry, Faculty of Science, University Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. Accepted 8 August, 2012 Petroleum ether extract of black pepper, Piper nigrum and physic nut, Jatropha curcas were shown to have insecticidal efficacies against rice moth, Corcyra cephalonica (Stainton). The C. cephalonica 3rd instar larvae were shown to have similarities susceptibility to petroleum ether extract of Piper nigrum and J. curcas with LC 50 values of 12.52 and 13.22 µL/ml, respectively. In a bioassay using no-choice tests, the parameters used to evaluate antifeedant activity were relative growth rate (RGR), relative consumption rate (RCR), efficiency on conversion of ingested food (ECI) and grain protection or feeding deterrence indices (FDI). Both extracts showed high bioactivity at all doses against C. cephalonica larvae and antifeedant action was increased with increasing plant extract concentrations. The petroleum ether extract of P. nigrum and J. curcas showed strong inhibition on egg hatchabilities and adult emergence of C. -

Evaluation of Mid-Gut Bacteria Present in the Larva of Rice Moth
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 9, ISSUE 03, MARCH 2020 ISSN 2277-8616 Evaluation Of Mid-Gut Bacteria Present In The Larva Of Rice Moth, Corcyra Cephalonica (Stain.) (Galleriidae: Lepidoptera) Fed On Different Grains Using 16S rDNA Sequence Based Culture Dependent Technique Arumugam Dhanalakshmi, Ganesan Sasireka, Palaniappan Suresh, Panagal Mani, Palanisamy Chella Perumal, Kalimuthu Ayyakalai Marikannu Karthikeyan* Abstract— Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) is a factitious host extensively used for rearing egg parasitoids. The present study attempt to investigate mid-gut bacteria present in larva of Corcyra cephalonica (C. cephalonica). The mid-gut bacteria were collected from the C. cephalonica fed on eight different grains were identified and characterized by 16S rDNA sequence based culture dependent method. The results revealed that, 16 bacterial species where presented in mid-gut of C. cephalonica larva which are belonging to 11 bacterial genera included in three phyla namely Firmicutes, Proteobacteria and Actinobacteria. Among the three phyla Firmicutes was the most dominant phylum with a record of 7 bacterial species, followed by Proteobacteria with 5 species and Actinobacteria with 4 species. Phylum Firmicutes, was dominated by members of Class Bacilli. The genus Staphylococcus was the largest genus represented by 4 species namely Staphylococcus sp., S. saprophyticus, Staphylococcus pasteuri and Staphylococcus warneri and also the most prevalent genus which was recorded in the mid-gut of C. cephalonica fed on 4 grains. No single bacterium was found in all the mid-gut samples. This record of varied mid-gut bacterial composition could be attributed to the feeding behaviour/pattern of the larvae and further intensive large scale research like extensive sampling and deep-sequencing are needed to ascertain it. -

View Full Text-PDF
Int.J.Curr.Res.Aca.Rev.2015; 3(4):373-380 International Journal of Current Research and Academic Review ISSN: 2347-3215 Volume 3 Number 4 ( April-2015) pp. 373-380 www.ijcrar.com Variation in biological parameters of Corcyra cephalonica Stainton (Lepidoptera:Pyralidae) with quality of rearing media N. Chaudhuri1*, A. Ghosh2 and J. Ghosh3 1Department of Agricultural Entomology, RRS, Terai Zone, Uttar Banga Krishi Viswavidyalaya, Pundibari, Cooch Behar-736165, West Bengal, India 2Department of Agricultural Statistics, RRS, Terai Zone, Uttar Banga Krishi Viswavidyalaya, Pundibari, Cooch Behar-736165, West Bengal, India 3Department of Agricultural Entomology, Uttar Banga Krishi Viswavidyalaya, Pundibari, Cooch Behar-736165, West Bengal, India *Corresponding author KEYWORDS ABSTRACT Food grains, Variation in biological parameters of Corcyra cephalonica Stainton dextrose, (Lepidoptera:Pyralidae) with quality rearing media was studied against four yeast, (4) different types of locally available grains namely maize, wheat, Italian larva, millet and scented rice as solo and fortified with 3% dextrose and yeast. The pupa, insect had significantly shortest larval-pupal period when the food grains adult, were fortified with dextrose followed by yeast and the period was longest in Corcyra solo grains. The insect complete its life cycle within 51.28 days during cephalonica August-October as compared to 54.67 days in May-August. The period was longest in scented rice (64.71 days) and shortest in maize-dextrose (45.00 days). Adult emergence was the maximum during August-October (71.33%) and in wheat (solo as well as fortified). The sex ratio showed female dominance. Fortification of 3% dextrose and yeast increased female per cent and thereby fecundity in Italian millet. -
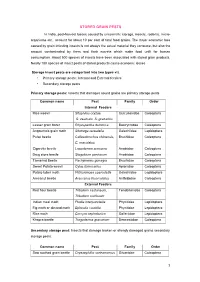
Stored Grain Pests
STORED GRAIN PESTS In India, post-harvest losses caused by unscientific storage, insects, rodents, micro- organisms etc., account for about 10 per cent of total food grains. The major economic loss caused by grain infesting insects is not always the actual material they consume, but also the amount contaminated by them and their excreta which make food unfit for human consumption. About 500 species of insects have been associated with stored grain products. Nearly 100 species of insect pests of stored products cause economic losses Storage insect pests are categorized into two types viz. • Primary storage pests : Internal and External feeders • Secondary storage pests Primary storage pests: Insects that damages sound grains are primary storage pests Common name Pest Family Order Internal Feeders Rice weevil Sitophilus oryzae, Curculionidae Coleoptera S. zeamais, S. granarius Lesser grain borer Rhyzopertha dominica Bostrychidae Coleoptera Angoumois grain moth Sitotroga cerealella Gelechiidae Lepidoptera Pulse beetle Callosobruchus chinensis, Bruchidae Coleoptera C. maculatus Cigarette beetle Lasioderma sericorne Anobiidae Coleoptera Drug store beetle Stegobium paniceum Anobiidae Coleoptera Tamarind Beetle Pachymeres gonagra Bruchidae Coleoptera Sweet Potato weevil Cylas formicarius Apionidae Coleoptera Potato tuber moth Phthorimoea operculella Gelechiidae Lepidoptera Arecanut beetle Araecerus fasciculatus Anthribidae Coleoptera External Feeders Red flour beetle Tribolium castaneum, Tenebrionidae Coleoptera Tribolium confusum Indian meal -

Mass Production Procedure of Trichogramma Spp
Mass production procedure of Trichogramma spp The genus Trichogramma is cosmopolitan in distribution and present in all terrestrial habitats and is one of 80 genera in the family Trichogrammatidae. Trichogramma primarily parasitise eggs of Lepidoptera, but parasitism also occurs in eggs of other orders such as Coleoptera, Diptera, Hemiptera, Hymenoptera and Neuroptera. It is important for plant protection because of its wide spread natural occurrence and its success as biological control agent by mass releasing. It has the distinction of being the highest produced and most utilized biological control agent in the world. Trichogrammatidae includes the smallest of insects, ranging in size from 0.2 to 1.5 mm. Biology of Trichogramma The development of all Trichogramma spp. is very similar. Being an egg parasite, the female drills a hole through the chorion and deposits its eggs within the egg of the host. The internal pressure of the egg forces a small drop of yolk out of the oviposition hole. Females feed on this yolk, which increases their longevity under laboratory conditions. Female parasitizes from one to ten eggs per day or from ten to 190 during her life. Large females parasitize more eggs than smaller females. The number of eggs laid per host egg may vary from 1 to 20 or more depending upon the size of the host egg. However, in sugarcane in which moth borer eggs are small, generally 1 or 2 parasites develop per egg. A female parasitoid can distinguish already parasitised eggs, thereby avoiding superparasitism or multiple-parasitism under natural conditions. Fecundity varies from 20 to 200 eggs per female according to the species, the host, and the longevity of the adult. -

A New Species of Galleria Fabricius (Lepidoptera, Pyralidae) from Korea
ZooKeys 970: 51–61 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.970.54960 RESEARCH ARTIclE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of Galleria Fabricius (Lepidoptera, Pyralidae) from Korea based on molecular and morphological characters Seung Jin Roh1, Haechul Park1, Seong-Hyun Kim1, So-Yun Kim1, Yong-Su Choi1, Jeong-Hun Song1 1 Department of Agricultural Biology, National Institute of Agricultural Sciences, Wanju 55365, South Korea Corresponding author: Jeong-Hun Song ([email protected]) Academic editor: Colin Plant | Received 8 June 2020 | Accepted 4 August 2020 | Published 21 September 2020 http://zoobank.org/8069F755-8DF6-4AEB-A1D7-FD20096B4C5C Citation: Roh SJ, Park H, Kim S-H, Kim S-Y, Choi Y-S, Song J-H (2020) A new species of Galleria Fabricius (Lepidoptera, Pyralidae) from Korea based on molecular and morphological characters. ZooKeys 970: 51–61. https:// doi.org/10.3897/zookeys.970.54960 Abstract The greater wax moth, Galleria mellonella Linnaeus, is well known as a pest of honey bees and for the biodegradation of wax and polyethylene by their larvae. The genus Galleria has long been considered monotypic and found worldwide. A taxonomic study of the genus Galleria is presented based on morpho- logical and molecular characters (COI, CAD, wg). A new species (Galleria similis Roh & Song, sp. nov.) is recognized on the Korean peninsula. The new species is superficially similar to G. mellonella but they can be separated by the structures of hindwing venation and male genitalia. Habitus photographs and illustra- tions of diagnostic characters are provided. Keywords cryptic species, Galleriinae, new species, plastic eating moth, Pyraloidea, wax worms Copyright Seung Jin Roh et al. -

Description of the Female of Aphomia Murciella (Lepidoptera: Pyralidae: Galleriinae)
ACTA ENTOMOLOGICA MUSEI NATIONALIS PRAGAE Published 15.xii.2014 Volume 54(2), pp. 785–788 ISSN 0374-1036 http://zoobank.org/urn:lsid:zoobank.org:pub:FA7CD654-6CA5-4C8F-8F5A-B74906EEFA3A Description of the female of Aphomia murciella (Lepidoptera: Pyralidae: Galleriinae) Jan ŠUMPICH Department of Entomology, National Museum, Cirkusová 1740, CZ-193 00 Praha 9 - Horní Poþernice, Czech Republic; e-mail: [email protected] Abstract. Aphomia murciella (Zerny, 1914) was described from several males originating from Murcia and it is known only from the Spanish mainland. Based on recent series from Andalucia, a female is described here for the ¿ rst time. Photographs of adults as well as genitalia of both sexes are provided. Key words. Lepidoptera, Pyralidae, Galleriinae, Aphomia murciella, female, Spain, Palaearctic Region Introduction The original description of Aphomia murciella (Zerny, 1914) is based on seven males collected in 1909 in the Sierra Espuña Mountains, Murcia province, Spain (ZERNY 1914). The type specimens and another male originating from Alfacar (Spain: prov. Granada) are deposited in the Natural History Museum, Vienna. So far, the only published ¿ ndings refer to the material mentioned above (SLAMKA 2006). Male genitalia of A. murciella were ¿ rst drawn by WHALLEY (1964) and recently published by SLAMKA (2006). Photographs of type adults were provided in the original description (ZERNY 1914) and later also by WHALLEY (1964) and SLAMKA (2006). A series of A. murciella containing specimens of both sexes from Andalucia, Spain, allowed me to describe the female here. Material and methods The presented material has been caught at light (UVA À uorescent tubes 8W) situated on stony slopes (Fig. -

A Theoretical Study on the Sex-Pheromones of the Rice Moth, Corcyra Cephalonica Stainton
Tr. J. of Biology 22 (1998) 229-232 © TÜBİTAK A Theoretical study on the Sex-pheromones of the Rice Moth, Corcyra cephalonica Stainton Lemi TÜRKER Middle East Technical University Department of Chemistry Ankara-TURKEY Recived: 08.05.1997 Abstract: Molecular orbital calculations (AM1 type) were performed on the geometry optimized structures of various farnesal isomers and methyl esters of farnesoic acids. It has been found that the major component, E, E-farnesal, of the sex-pheromone for the rice moth, Corcyra cephalonica Stainton is chemically the most exothermic and stable of all the farnesal isomers. Then, the resem- blance of molecular shapes was detrmined in order to design more stable sex-attractants. Key Words: Corcyra cephalonica, Pyralidae, Galleriinae, molecular orbital calculations, pheromones. Prinç Güvesi Corcyra cephalonica Stainton’un Seks Feromonları Üzerine Teorik bir Araştırma Özet: Geometry optimize edilmiş çeşitli farnesal izomerleri ve farnesoic acid metil esterleri üzerinde moleküler yörünge hesaplamaları (AM1 tipi) yapılmıştır. Pirinç güvesi, Corcyra cephalonica. Stainton’un seks-feromonunun esas elemanı olan E, E-farnesal’ın, bütün farnesal izomerleri içinde kimyasal olarak en ekzotermik ve dayanaklı olduğu bulunmuştur. Müteakiben, daha dayanıklı seks-çekicileri tasarımı amacı ile, molekülsel şekil benzerlikleri tayin edilmiştir. Anahtar Sözcükler: Corcyra cephalonica, Pyralidae. Galleriinae, moleküler yörünge hesapları, fer- omonlar. Introduction The rice moth, Corcyra cephalonica Stainton (Pyralidae, Galleriinae) is a major pest of stored products and quite often its population thus its harm reaches very unplesant level in subtropical countries like India that, some extensive research has been carried out about the biology of this insect as well as different aspects of the sex pheromone (1, 2).