Limited Value of the Common Periwinkle Snail Littorina Littorea As a Biological Control for the Invasive Tunicate Didemnum Vexillum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Characterisation of the Populations and Immune-Related
First characterisation of the populations and immune-related activities of hemocytes from two edible gastropod species, the disk abalone, Haliotis discus discus and the spiny top shell, Turbo cornutus. Ludovic Donaghy, Hyun-Ki Hong, Christophe Lambert, Heung-Sik Park, Won Joon Shim, Kwang-Sik Choi To cite this version: Ludovic Donaghy, Hyun-Ki Hong, Christophe Lambert, Heung-Sik Park, Won Joon Shim, et al.. First characterisation of the populations and immune-related activities of hemocytes from two edible gastropod species, the disk abalone, Haliotis discus discus and the spiny top shell, Turbo cornutus.. Fish and Shellfish Immunology, Elsevier, 2010, 28 (1), pp.87-97. 10.1016/j.fsi.2009.10.006. hal- 00460531 HAL Id: hal-00460531 https://hal.archives-ouvertes.fr/hal-00460531 Submitted on 1 Mar 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. First characterisation of the populations and immune-related activities of hemocytes from two edible gastropod species, the disk abalone, Haliotis discus discus and the spiny top shell, Turbo cornutus . Ludovic Donaghy a,b,* , Hyun-Ki Hong a, Christophe Lambert b, Heung-Sik Park c, Won Joon Shim d, Kwang-Sik Choi a. -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1. -
Show Ethnoactivity
U Emetic Plant Abrus precatorius (Paternoster; Rakat; Reglisse; Pois Rouge; Weesboontje; Ma Liao Tou; Jequerit; Hung Tou; Graines Reglisse; Peonia De St Tomas; Paratella; Peonia; Hint Meyankoku; Liane Reglisse; Gunchi; Rosary Pea; To-Azuki; Cain Ghe) Acacia farnesiana (Tusca; Kembang bandira; Cuji; Esponjeira; Kambang japun; Kembang nagasiri) Acalypha indica (Lelatang) Achyranthes aspera (Apamarga; Rarai; Feuilles La Fievre; Rabo De Gato; Chaff Tree; Jarongan; Santypite) Acorus calamus (Jerangau; Calomo Aromatico; Sweet Flag; Vacha; Ganoeak; Doringo; Seki-Sho; Jeringau; Bach; Djerango; Shui Ch'Ang Pu; Acoro Aromatico; Vaj; Kalmus; Calamus; Ch'Ang P'U Chiu; Agri Turki; Kalmoes; Calmus; Acore Vrai; Acorus; Sarango; Jariangau; Calamo Aromatico; Azakegeri; Kalmos; Kaliraga; Sho-Bu) Adenocalymna alliaceum Aesculus argutus Ageratum conyzoides (Rompesaraguelo; Rumput tahi ayam; Wedusan; Berokan; Aru batu; Bandotan) Ailanthus altissima (Gok Aghaji; Ch'Ou Ch'Uang Shu Ken; Ch'U; Lisan At Tair; Niwa-Urusi) Ailanthus glandsulosa (Kokar Agac) Alangium salviifolium Alchornea cordifolia Aletris farinosa (Sterwortel; Unicorne; Sternwurzel; Star Grass; Blazing Star; True Unicorn) Aleurites fordii (Jabilla Extranjera; Tung) Allamanda blanchetii Allamanda cathartica (Yellow Allamanda) Alnus rubra (Rode Els; Aune Rouge; Red Alder; Rote Erle) Alnus rugosa Alpinia malaccensis Amaranthus dubius (Zepina; Epinard Marron; Bledo) Ammi visnaga (Khellakraut; Khillah; Busnaga; Pick Toothh; Viznaga; Khaizaran; Biznaga; Anmi; Bisnaga Das Searas) Anagyris foetida (Domuzdikeni; -
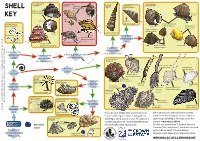
Shell Whelk Dog Whelk Turret It Could Be a Periwinkle Shell (Nucella Lapillus) Shell Spire Shell Thick Top Shell (Osilinus Lineatus) Dark Stripes Key on Body
It could be a type of It could be a type of It could be a It could be a type of topshell whelk Dog whelk turret It could be a periwinkle Shell (Nucella lapillus) shell spire shell Thick top shell (Osilinus lineatus) Dark stripes Key on body Egg Underside capsules Actual size It could be a type of (Hydrobia sp) Common periwinkle spiral worm White ‘Colar’ (Littorina littorea) Flat periwinkle (Littorinasp) Yes Roughly ‘ribbed’ shell. Very high up shore ‘Tooth inside (Turitella communis) opening (Spirorbis sp) Does it have 6 Common whelk No (Buccinum undatum) Yes or more whorls Brown, speckled Netted dog whelk body (twists)? Painted topshell (Nassarius reticulatus) (Calliostoma zizyphinum) No Rough periwinkle Flattened spire Yes Is it long, thin (Littorina saxatilis) Yes Yes and cone shaped Is it permanently No like a unicorn’s horn? attached to Is there a groove or teeth No Is there mother No a surface? in the shell opening? of pearl inside It could be a type of the shell opening? bivalve Yes Yes Common otter-shell (Lutraria lutraria) Bean-like tellin No Is the shell in (Fabulina fabula) Is it 2 parts? spiraled? Common cockle (Cerastoderma edule) It could be a Flat, rounded No sand No Great scallop mason It could be a Is the shell a (Pecten maximus) shell Razor shell worm keel worm Wedge-shaped Is the case dome or (Ensis sp) No Pacific oyster shell made from Yes cone shape? (Crassostrea gigas) Shell can be Peppery furrow shell very large (Scrobicularia plana) sand grains? Elongated and and doesn’t (Lanice conchilega) deep-bodied fully close with large ‘frills’ No (Pomatoceros sp) Yes It could be a type of sea urchin It could be a type of An acorn Native oyster Empty barnacle barnacle Does it have that may be found in estuaries and shores in the UK. -

Impact of Trematode Parasitism on the Fauna of a North Sea Tidal Flat
HELGOI~NDER MEERESUNTERSUCHUNGEN Helgol~nder Meeresunters. 37, 185-199 (1984) Impact of trematode parasitism on the fauna of a North Sea tidal flat G. Lauckner Biologische Anstalt Helgoland (Litoralstation]; D-2282 List/Sylt, Federal Republic of Germany ABSTRACT: The impact of larval trematodes on the fauna of a North Sea tidal flat is considered at the individual and at the population level, depicting the digenean parasites of the common periwinkle, Littorina littorea, and their life cycles, as an example. On the German North Sea coast, L. fittorea is first intermediate host for 6 larval trematodes representing 6 digenean families - Cryptocotyle lingua (Heterophyidae), Himasthla elongata (Echinostomatidae), Renicola roscovita (Renicolidae), Microphallus pygmaeus (Microphallidae), Podocotyle atomon (Opecoelidae} and Cercaria lebouri (Notocotylidae). All except P. atomon utilize shore birds as final hosts; adult P. atomon parasitize in the intestine of teleosts, mainly pleuronectid flatfish. Second intermediate hosts of C. lingua are various species of fish; the cercariae of H. elongata encyst in molluscs and polychaetes, those of R. roscovita in molluscs; Iv[. pygmaeus has an abbreviated life cycle; C. lebouri encysts free on solid surfaces; and the fish trematode P. atomon utilizes benthic crustaceans, mainly amphipods, as second intermediate hosts. On the tidal flats of the K6nigshafen (Sylt), up to 77 % of the periwinkles have been found to be infested by larval trematodes. Maximum infestations in individual samples were 23 % for C. lingua, 47 % for H. etongata and 44 To for R. roscovita. The digeneans cause complete 'parasitic castration' of their carriers and hence exclude a considerable proportion of the snails from the breeding population. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Click Here to Download
Interested in European research? RTD info is our quarterly magazine keeping you in touch with main developments (results, programmes, events, etc.). It is available in English, French and German. A free sample copy or free subscription can be obtained from: Directorate-General for Research Communication Unit European Commission Rue de la Loi/Wetstraat 200 B-1049 Brussels Fax (32-2) 29-58220 E-mail: [email protected] Internet: http:/europa.eu.int/comm/research/rtdinfo.html EUROPEAN COMMISSION Directorate-General for Research Unit AP.2 - COST Contact: Mrs Marija Skerlj Address: European Commission, rue de la Loi/Wetstraat 200 (SDME 1/43), B-1049 Brussels - Tel. (32-2) 29-91599; fax (32-2) 29-65987 European Commission COST Action 99 Research action on food consumption and composition data LanguaL 2000 The LanguaL thesaurus Working Group on food description, terminology and nomenclature Edited by: Anders Møller and Jayne Ireland Directorate-General for Research 2000 EUR 19540 LEGAL NOTICE Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of the following information. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int). Cataloguing data can be found at the end of this publication. Luxembourg: Office for Official Publications of the European Communities, 2000 ISBN 92-828-9758-3 © European Communities, 2000 Reproduction is authorised provided the source is acknowledged. Printed in Belgium PRINTED ON WHITE CHLORINE-FREE PAPER LanguaL 2000 THE LANGUAL THESAURUS PREPARED BY ANDERS MØLLER JAYNE IRELAND ACKNOWLEDGEMENTS We would like to express our gratitude to the Center for Food Safety and Applied Nutrition (CFSAN) of the United States Food and Drug Administration (FDA) for so willingly sharing all the information concerning LanguaL and other indexing systems at the FDA. -

Lipids and Fatty Acids of Sea Hares Aplysia Kurodai and Aplysia Juliana
Journal of Oleo Science Copyright ©2019 by Japan Oil Chemists’ Society doi : 10.5650/jos.ess19137 J. Oleo Sci. 68, (12) 1199-1213 (2019) Lipids and Fatty Acids of Sea Hares Aplysia kurodai and Aplysia juliana: High Levels of Icosapentaenoic and n-3 Docosapentaenoic Acids Hiroaki Saito1, 2* and Hisashi Ioka1† 1 SA Lipid Laboratory, 2-1-12, Koyanagi, Aomori 030-0915, JAPAN 2 Japan Inspection Institute of Fats and Oils, 1-8-2, Shinobashi, Koto-ku, Tokyo 135-0007, JAPAN † Present address, Shimane Prefectural Fisheries Technology Center, Hamada 697-0051, Shimane, JAPAN Abstract: The lipid and fatty acid compositions of two species of gastropods, Aplysia kurodai and Aplysia juliana (collected from shallow sea water), were examined to assess their lipid profiles, health benefits, and the trophic relationships between herbivorous gastropods and their diets. The primary polyunsaturated fatty acids (PUFAs) found in the neutral lipids of all gastropod organs consisted of four shorter chain n-3 PUFAs: linolenic acid (LN, 18:3n-3), icosatetraenoic acid (ITA, 20:4n-3), icosapentaenoic acid (EPA, 20:5n- 3), and docosapentaenoic acid (DPA, 22:5n-3). The PUFAs found in polar lipids were various n-3 and n-6 PUFAs: arachidonic acid (ARA, 20:4n-6), adrenic acid (docosatetraenoic acid, DTA, 22:4n-6), icosapentaenoic acid (EPA, 20:5n-3), and docosapentaenoic acid (DPA, 22:5n-3) in addition to trace levels of docosahexaenoic acid (DHA, 22:6n-3). Various n-3 and n-6 PUFAs (18:2n-6, 20:2n-6, 18:3n-6, 20:3n-6, 18:3n-3, 18:4n-3, 20:3n-3, n-3 ITA, and 22:3n-6,9,15) comprised the biosynthetic profiles of A. -

Dr. Duke's Phytochemical and Ethnobotanical Databases Ehtnobotanical Plants for Emetic
Dr. Duke's Phytochemical and Ethnobotanical Databases Ehtnobotanical Plants for Emetic Ehnobotanical Plant Common Names Abrus precatorius Gunchi; Liane Reglisse; Hint Meyankoku; Hung Tou; Ma Liao Tou; Rosary Pea; Cain Ghe; Jequerit; To- Azuki; Paternoster; Pois Rouge; Peonia De St Tomas; Graines Reglisse; Weesboontje; Reglisse; Rakat; Paratella; Peonia Acacia farnesiana Tusca; Kembang bandira; Kambang japun; Kembang nagasiri; Esponjeira; Cuji Acalypha indica Lelatang Achyranthes aspera Santypite; Rarai; Chaff Tree; Apamarga; Jarongan; Feuilles La Fievre; Rabo De Gato Acorus calamus Calamo Aromatico; Acore Vrai; Seki-Sho; Ch'Ang P'U Chiu; Djerango; Calomo Aromatico; Acoro Aromatico; Doringo; Kalmos; Azakegeri; Jeringau; Sho-Bu; Jerangau; Kaliraga; Bach; Calamus; Kalmus; Kalmoes; Shui Ch'Ang Pu; Vaj; Sweet Flag; Acorus; Sarango; Agri Turki; Vacha; Ganoeak; Jariangau; Calmus Adenocalymna alliaceum Aesculus argutus Ageratum conyzoides Bandotan; Wedusan; Rumput tahi ayam; Aru batu; Rompesaraguelo; Berokan Ailanthus altissima Lisan At Tair; Gok Aghaji; Niwa-Urusi; Ch'U; Ch'Ou Ch'Uang Shu Ken Ailanthus glandsulosa Kokar Agac Alangium salviifolium Alchornea cordifolia Aletris farinosa Blazing Star; Star Grass; Sterwortel; Unicorne; Sternwurzel; True Unicorn Aleurites fordii Tung; Jabilla Extranjera Allamanda blanchetii Allamanda cathartica Yellow Allamanda Alnus rubra Rode Els; Aune Rouge; Red Alder; Rote Erle Alnus rugosa Alpinia malaccensis Amaranthus dubius Epinard Marron; Zepina; Bledo Ammi visnaga Khillah; Pick Toothh; Khellakraut; Khaizaran; -

Macoma Balthica
+ model ARTICLE IN PRESS Journal of Experimental Marine Biology and Ecology xx (2005) xxx–xxx www.elsevier.com/locate/jembe Chemically induced predator avoidance behaviour in the burrowing bivalve Macoma balthica C.L. Griffiths a, C.A. Richardson b,* a Zoology Department and Marine Biology Research Institute, University of Cape Town, Rondebosch 7701, South Africa b School of Ocean Sciences, University of Wales Bangor, Menai Bridge, Anglesey, LL59 5AB, UK Received 4 July 2005; received in revised form 4 October 2005; accepted 10 October 2005 Abstract The responses of the burrowing bivalves Macoma balthica and Cerastoderma edule to chemical cues emitted by feeding shore crabs Carcinus maenas were investigated. M. balthica held in the laboratory and exposed to chemical signals in effluent water discharging from tanks containing C. maenas fed 20 M. balthica dayÀ 1 reacted by increasing their burial depths from approximately 30 mm to depths of N60 mm, over a period of several days. When the signal was removed the bivalves gradually returned to their original depth over 5 days. C. edule similarly exposed to effluent from crabs feeding on conspecifics showed no response. In an attempt to identify the signal inducing this burrowing response, M. balthica were exposed to a variety of chemical signals. Crabs fed M. balthica elicited the strongest response, followed by crabs fed C. edule. There were also small responses to effluent from crabs fed on fish, crabs previously fed on M. balthica and to crab faeces, but no responses to starved crabs, crushed M. balthica, or controls. We conclude that increased burrowing depth of M. -

Biolphilately Vol-64 No-3
Vol. 66 (1) Biophilately March 2017 65 MARINE INVERTEBRATES Editor Ian Hunter, BU1619 New Listings Scott# Denom Common Name/Scientific Name Family/Subfamily Code ALBANIA 2015 December 23 (Underwater Fauna) (Horiz pair & SS/1) 2980a 5l Mediterranean Fan Worm, Sabella spallanzanii Sabellidae A 2980b 150l Mediterranean Feather Star, Antedon mediterranea Antedonidae A 2981 SS 250l Mediterranean Jelly Fish, Cotylorhiza tuberculata Cepheidae A AUSTRALIA 2016 March 1 (Items Starting with Same Letter) (Horiz strip/5 & Bklt) 4454 $1 Surf, surfboard, sand castle, starfish, sausage, shark, toy, shell, snail, map S C 4459 $1 Like Sc#4454 (die cut 11¼ syncopated) (s/a) S C 4459a Bklt/10 (Sc#4459) BOSNIA & HERZEGOVINA 2016 August 5 (Summer Sports & Recreation) 768 2.50m Stylized starfish, shell, crab, dolphins, and fish in beach scene S C BOSNIA & HERZEGOVINA (Serb) 2016 October 14 (River Fauna) (Set/5) 552 1m European Crayfish, Astacus astacus Astacidae A BHUTAN 2016 (Auspicious Symbols) (MS/8) 1546g 30nu Indian Chank Shell, Turbinella pyrum Turbinellidae A CHRISTMAS ISLAND 2016 October 31 (Christmas) (SS/2) 553a Margin Bot: Stylized Crabs S Z DJIBOUTI 2016 June 6 (Shells) 1034a 260fr Red Helmet Shell, Cypraecassis rufa Cassidae A 1034b 260fr Triton’s Trumpet, Charonia tritonis Ranellidae A 1034c 260fr a Top Shell, Trochus nodulosus Trochidae A 1034d 260fr Ermine Cone, Conus mustelinus Conidae A 1053 SS 960fr Spider Conch, Lambis lambis Strombidae A Margin Top: Beaded Prickly Winkle, Tectarius coronatus Littorinidae Z Ctr & R: Common Periwinkle, Littorina -

Plant Invaders of Mid-Atlantic Natural Areas Revised & Updated – with More Species and Expanded Control Guidance
Plant Invaders of Mid-Atlantic Natural Areas Revised & Updated – with More Species and Expanded Control Guidance National Park Service U.S. Fish and Wildlife Service 1 I N C H E S 2 Plant Invaders of Mid-Atlantic Natural Areas, 4th ed. Authors Jil Swearingen National Park Service National Capital Region Center for Urban Ecology 4598 MacArthur Blvd., N.W. Washington, DC 20007 Britt Slattery, Kathryn Reshetiloff and Susan Zwicker U.S. Fish and Wildlife Service Chesapeake Bay Field Office 177 Admiral Cochrane Dr. Annapolis, MD 21401 Citation Swearingen, J., B. Slattery, K. Reshetiloff, and S. Zwicker. 2010. Plant Invaders of Mid-Atlantic Natural Areas, 4th ed. National Park Service and U.S. Fish and Wildlife Service. Washington, DC. 168pp. 1st edition, 2002 2nd edition, 2004 3rd edition, 2006 4th edition, 2010 1 Acknowledgements Graphic Design and Layout Olivia Kwong, Plant Conservation Alliance & Center for Plant Conservation, Washington, DC Laurie Hewitt, U.S. Fish & Wildlife Service, Chesapeake Bay Field Office, Annapolis, MD Acknowledgements Funding provided by the National Fish and Wildlife Foundation with matching contributions by: Chesapeake Bay Foundation Chesapeake Bay Trust City of Bowie, Maryland Maryland Department of Natural Resources Mid-Atlantic Invasive Plant Council National Capital Area Garden Clubs Plant Conservation Alliance The Nature Conservancy, Maryland–DC Chapter Worcester County, Maryland, Department of Comprehensive Planning Additional Fact Sheet Contributors Laurie Anne Albrecht (jetbead) Peter Bergstrom (European