Selective Equatorial Sclera Crosslinking in the Orbit Using a Metal-Coated Polymer Waveguide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

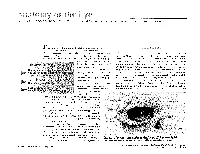
MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -
The Orbit Is Composed Anteri
DAVID L. PARVER, MD The University of Texas Southwestern Medical Center, Dallas Theability to successfully assess and treat The Orbit physical ailments requires an understanding of the anatomy involved in the injury or The eye itself lies within a protective shell trauma. When dealing with injuries and called the bony orbits. These bony cavities are trauma associated with the eye, it is neces- located on each side of the root of the nose. sary to have a work- Each orbit is structured like a pear with the ing knowledge of optic nerve, the nerve that carries visual im- basic ocular anatomy pulses from the retina to the brain, represent- so that an accurate ing the stem of the orbtt (Duke-Elder, 1976). Understa eye also diagnosis can be Seven bones make up the bony orbit: frontal, achieved and treat- zygomatic, maxillary, ethmoidal, sphenoid, ment can be imple- lacrimal, and palatine (Figures 1 and 2). in a bony " mented. The roof of the orbit is composed anteri- . .. The upcoming ar- orly of the orbital plate of the frontal bone ticles in this special and posteriorly by the lesser wing of the sphe- Each portion of the 01 I noid bone. The lateral wall is separated from .r. theme section the nervc an eye will deal specifically 2 with recognizing ocular illness, disease, and injuries, and will also address the incidence of sports related eye injuries and trauma. This paper covers the ba- sics of eye anatomy, focusing on the eye globe and its surrounding struc- tures. Once one gains an understand- ing of the normal anatomy of the eye, it will be easier to recognize trauma, injury, or illness. -

Evisceration, Enucleation and Exenteration
CHAPTER 10 EVISCERATION, ENUCLEATION AND EXENTERATION This chapter describes three operations that either remove the contents of the eye (evisceration), the eye itself (enucleation) or the whole orbital contents (exenteration). Each operation has specific indications which are important to understand. In many cultures the removal of an eye, even if blind, is resisted. If an eye is very painful or grossly disfigured an operation will be accepted more readily. However, if the eye looks normal the patient or their family may be very reluctant to accept its removal. Therefore tact, compassion and patience are needed when recommending these operations. ENUCLEATION AND EVISCERATION There are several reasons why either of these destructive operations may be necessary: 1. Malignant tumours in the eye. In the case of a malignant tumour or suspected malignant tumour the eye should be removed by enucleation and not evisceration.There are two important intraocular tumours, retinoblastoma and melanoma and for both of them the basic treatment is enucleation. Retinoblastoma is a relatively common tumour in early childhood. At first the growth is confined to the eye. Enucleation must be carried out at this stage and will probably save the child’s life. It is vital not to delay or postpone surgery. If a child under 6 has a blind eye and the possibility of a tumour cannot be ruled out, it is best to remove the eye. Always examine the other eye very carefully under anaesthetic as well. It may contain an early retinoblastoma which could be treatable and still save the eye. Retinoblastoma spreads along the optic nerve to the brain. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. -

MORPHOMETRIC and MORPHOLOGICAL ANALYSIS of FORAMEN OVALE in DRY HUMAN SKULLS Ashwini
International Journal of Anatomy and Research, Int J Anat Res 2017, Vol 5(1):3547-51. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2017.109 MORPHOMETRIC AND MORPHOLOGICAL ANALYSIS OF FORAMEN OVALE IN DRY HUMAN SKULLS Ashwini. N.S *1, Venkateshu. K.V 2. *1 Assistant Professor, Department Of Anatomy,Sri Devaraj Urs Medical College ,Tamaka, Kolar, Karnataka, India. 2 Professor And Head, Department Of Anatomy ,Sri Devaraj Urs Medical College, Tamaka, Kolar, India. ABSTRACT Introduction: Foramen ovale is an important foramen in the middle cranial fossa. Foramen ovale is situated in the greater wing of sphenoid bone, posterior to the foramen rotandum. Through the foramen ovale the mandibular nerve, accessory meningeal artery, lesser petrosal nerve, emissary veins pass .The shape of the foramen ovale is usually oval compared to other foramen of the skull. Materials and Methods: The study was conducted on 55 dry human skulls(110 sides) in Department of Anatomy, Sri Devaraj Urs Medical college, Tamaka, Kolar. Skulls in poor condition or skulls with partially damaged surroundings around the foramen ovale were excluded from the study. Linear measurements were taken on right and left sides of foramen ovale using divider and meter rule. Results: The maximum length of foramen ovale was 14 mm on the right side and 17 mm on the left, its maximum breadth on the right side was 8mm and 10mm on the left . Through the statistical analysis of morphometric measurements between right and left foramen ovale which was found to be insignificant , the results of both sides marks as the evidence of asymmetry in the morphometry of the foramen ovale. -

Primary Tumors of the Canine! Conjunctiva, Eyelids and Orbit! "
Primary Tumors of the Canine! Conjunctiva, Eyelids and Orbit! " Richard R Dubielzig" Anatomic Distribution of Canine Primary Ocular Neoplasia (n = 6110) Orbit, 285, 5% Orbit! 288! 6%! Eyelid, 1408, 23% Globe, 3225, 53% Conjunctiva, 1192, 19% Canine Primary Conjunctival Tumors Not (n = 1192) Conjunctiva, 4918, 80% Conjunctiva, Peripheral Nerve Sheath Tumor 11 1192, 20% Viral Papilloma 39 Tumors of the conjunctiva = Mast Cell Tumor 52 1192 of 6110 total neoplasms, Squamous Cell Carcinoma 85 ~ 20%! Squamous Papilloma 110 Tumor of the Third Eyelid Gland 152 Hemangiosarcoma 156 Hemangioma 163 Melanoma 204 Reactive Papilloma 220 0 50 100 150 200 250 Conjunctival Melanoma" • 293 cases" • Most are malignant by morphologic criteria" • Most recur elsewhere on the conjunctiva when removed" • They are less likely to metastasize than recur" • Those on the palpebral conjunctiva are more likely to metastasize" Conjunctival Melanoma" Conjunctival Melanoma! typically multifocal distribution! Conjunctival Melanoma! pagetoid spread within the epithelium! Conjunctival Melanoma! pagetoid spread within " the epithelium! Melan A! Intraepithelial Pagetoid spread! Canine Conjunctival Squamous Papilloma" •183 cases in the COPLOW database" •25 Golden Retriever" •24 Mixed breed" •12 Shih Tzu" •11 Labrador Retriever" •Characterized by pointed fronds of epithelium" •No underlying inflammation or neoplasia" •These are seldom correctly diagnosed clinically" Canine Conjunctival Reactive Papilloma" •Most often associated with " tumors or inflammation, hence" reactive" Canine -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos. -

Skull Base Tumors Involving the Orbit
Skull Base Tumors Involving the Orbit Donald J. Annino, Jr, MD, DMD a a SkullSkull BaseBase TumorsTumors ChallengingChallenging toto treattreat RareRare MultipleMultiple histologieshistologies ComplexComplex anatomyanatomy OrbitOrbit –– BonyBony AnatomyAnatomy KeyKey structurestructure middlemiddle 1/31/3 faceface 77 bonesbones CommunicatesCommunicates withwith anterior,anterior, middlemiddle cranialcranial fossae,fossae, infratemporalinfratemporal andand pterygopalatinepterygopalatine fossaefossae SuperiorSuperior andand inferiorinferior orbitalorbital fissuresfissures SkullSkull BaseBase TumorsTumors InvolvingInvolving thethe OrbitOrbit PrimaryPrimary SecondarySecondary MetastaticMetastatic PrimaryPrimary OrbitalOrbital TumorsTumors BenignBenign -- InflammatoryInflammatory VascularVascular –– CavernousCavernous hemangiomashemangiomas NerveNerve sheathsheath BonyBony LymphangiomaLymphangioma PrimaryPrimary MalignantMalignant LacrimalLacrimal glandgland 5050 %% malignantmalignant ACC,ACC, malignantmalignant mixedmixed LacrimalLacrimal sacsac OsteosarcomaOsteosarcoma –– afterafter retinoblastomaretinoblastoma RhabdomyosarcomaRhabdomyosarcoma SecondarySecondary OrbitalOrbital TumorsTumors ParanasalParanasal sinusessinuses IntracranialIntracranial MeningiomasMeningiomas SkinSkin ParanasalParanasal SinusSinus LesionsLesions BenignBenign OsteomasOsteomas MucoceleMucocele PolypsPolyps MalignantMalignant CarcinomaCarcinoma SarcomaSarcoma MucosalMucosal melanomamelanoma ParanasalParanasal SinusSinus TumorTumor ~50 -

Age-Related Changes of the Orbit and Midcheek and the Implications for Facial Rejuvenation
Aesth. Plast. Surg. 30:1À6, 2007 DOI: 10.1007/s00266-006-0120-x Original Article Age-Related Changes of the Orbit and Midcheek and the Implications for Facial Rejuvenation Bryan C. Mendelson,1 Winfield Hartley,1 Mark Scott,1 Alan McNab,1 and Jay W. Granzow2 1 1109 Mathoura Road, Toorak 3142, Melbourne, Victoria, Australia 2 2UCLA Division of Plastic Surgery, Los Angeles, CA, USA Abstract. Facial aging is a complex and incompletely under- 4 Background:Aging of the midface is complex and poorly stood process. In the past, most authors focused on understood. Changes occur not only in the facial soft tis- changes in the facial soft tissues and skin to explain sues, but also in the underlying bony structure. Computed the process of facial aging. However, it has been tomography (CT) imaging was used for investigating shown that changes in the bony facial skeleton also characteristics of the bony orbit and the anterior wall of the contribute significantly to the alterations in facial maxilla in patients of different ages and genders. appearance that occur with increasing age. As first Methods: Facial CT scans were performed for 62 patients proposed by Enlow [2], these bony changes occur ranging in age from 21 to 70 years, who were divided into differently in different parts of the face [1,3,6À10,14]. three age groups: 21À30 years, 41À50 years, and 61À70 The inferior orbital rim and the anterior maxilla years. Patients also were grouped by gender. The lengths of serve as a key foundation for the soft tissues of the the orbital roof and floor and the angle of the anterior wall inferior orbit and midface. -

Orbital Meningiomas Meningiomas Orbitários Carlos Eduardo Da Silva, M.D
31 Revisão Orbital Meningiomas Meningiomas Orbitários Carlos Eduardo da Silva, M.D. 1 Paulo Eduardo Freitas, M.D. Ph.D.2 Alicia Del Carmem Becerra Romero, M.D.3 Tâmen Moyses Pereyra4 Vicente Faraon Fonseca4 Willian Alves Martins4 Márcio Aloisio Bezerra Cavalcanti Rockenbach4 Fáberson João Mocelin Oliveira4 ABSTRACT RESUMO Orbital meningiomas usually invade the orbit as an extension Meningeomas orbitários invadem a órbita, na maioria dos ca- of the sphenoid wing meningiomas, clinoidal meningiomas, sos, como uma extensão de meningeomas da asa do esfenóide, cavernous sinus meningiomas and tuberculum sella tumors. meningeomas do seio cavernoso, meningeomas da clinóide e They also arise into the orbit originating from the optic sheath do tubérculo da sela. Eles também podem ser originados do or as ectopical lesions. The authors present a review of clini- revestimento dural do nervo óptico ou como lesões ectópicas cal aspects and surgical treatment of the orbital meningio- intraorbitais. Os autores apresentam uma revisão dos aspectos mas. Material and methods: The authors present a literature clínicos e cirúrgicos dos meningeomas orbitários. Material e review of the anatomical, clinical, and surgical aspects of métodos: Os autores apresentam uma revisão da literatura dos the orbital meningiomas, add illustrative cases, pointing aspectos anatômicos, clínicos e cirúrgicos dos meningeomas their principal concerns about the treatment of such tumors. orbitários, com casos ilustrativos, apresentando suas principais Results: Exophthalmos and unilateral visual loss are the most preocupações no manejo destes tumores. Resultados: Exoftal- common features of the orbital meningiomas. There are two mia e perda visual unilateral são os achados mais frequentes important surgical routes to approach such tumors, which are nos meningeomas orbitários. -

The Role of the Sclera and Orbital Tissues in the Biomechanical Deformation Response of the Cornea and Whole Eye Under Loading B
The Role of the Sclera and Orbital Tissues in the Biomechanical Deformation Response of the Cornea and Whole Eye Under Loading by Dynamic Scheimpflug Analyzer Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Boihoan Audrey Nguyen, M.S. Graduate Program in Biomedical Engineering The Ohio State University 2019 Dissertation Committee Cynthia J. Roberts, Ph.D, Advisor Matthew A. Reilly, Ph.D., Co-advisor Jun Liu, Ph.D., Committee Member Copyrighted by Boihoan Audrey Nguyen 2019 2 Abstract The biomechanical behavior of the ocular and orbital tissues are important to maintaining proper structure and function of the eye. The cornea and the sclera are the two main components of the ocular shell, which are loaded by IOP and are responsible for maintaining the structure and therefore function of the eye. This work comprises of four studies which explore the contribution of the sclera and the biomechanical response of the eye. The first study focused on the development of a finite-element model to explore the impact of varying scleral properties on the deformation response of the cornea to an air- puff. An axisymmetric model of the eye – consisting of a cornea, sclera, and vitreous humor – loaded internally by intraocular pressure (IOP) and externally by an air-puff from a noncontact tonometer was generated in COMSOL 5.2a. Our results showed that increasing scleral stiffness (with constant corneal stiffness and IOP) resulted in decreasing displacement of the corneal apex, i.e. the cornea responded to the air-puff as if it had stiffer mechanical properties. -

Table 1-1 Bones of the Orbit
6 ● Neuro-Ophthalmology Planum sphenoidale Superior orbital fissure Optic canal Tuberculum sella Cavernous Anterior clinoid Pituitary sinus fossa Foramen ovale Carotid canal Foramen spinosum Foramen lacerum Clivus Petrous portion Dorsum sella of temporal bone Figure 1-3 Parasellar bony anatomy demonstrates the relationship of the pituitary fossa to the cavernous sinus, including the foramina of the skull base. The foramen lacerum is filled with cartilage and contains the artery of the pterygoid canal, the nerve of the pterygoid canal, and the venous drainage structures. The carotid artery enters the skull base through the carotid canal. (Courtesy of Albert L. Rhoton Jr, MD.) Frontal bone Lesser wing of sphenoid bone Superior orbital Optic canal fissure Greater wing of Ethmoidal bone sphenoid bone Lacrimal bone Zygomatic bone Lacrimal sac Palatine bone fossa Inferior orbital Maxillary bone fissure Figure 1-4 Anatomy of the orbit. Bony anatomy of the right orbital apex. The optic canal trans- mits the optic nerve, ophthalmic artery, and some oculosympathetic fibers. The superior orbital fissure, between the greater and lesser wings of the sphenoid bone, transmits CNs III, IV, and VI; the ophthalmic division of CN V (CN V1); the oculosympathetics; and the superior ophthalmic vein. (Illustration by Dave Peace.) Table 1-1 Bones of the Orbit Orbital Roof Lateral Wall Orbital Floor Medial Wall Frontal Zygomatic Zygomatic Maxillary Lesser wing of sphenoid Greater wing of sphenoid Maxillary Lacrimal Palatine Ethmoid Lesser wing of sphenoid CHAPTER 1: Neuro- Ophthalmic Anatomy ● 7 The superior orbital rim is made up of the frontal bone, which connects to the zygomatic bone laterally at the frontozygomatic suture.