Leaf Variegation in Caladium Steudneriifolium (Araceae): a Case of Mimicry?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LD5655.V855 1924.M377.Pdf
A STUDY OF BACILLUS AROIDEAE 'l'OWNSEND, THE CAUSE OF A SOFT ROT OF TOMATO, AN:) B. CAROTOVORUS JONES. A thesis 811.bmitted as partial requirement for the degree of Master of Science in Botany by A.B.Massey Reprinted from PHYT0PATH0L0GY, Vol. XIV, October, 1924. A STUDY OF BACILLUS AROIDEAE, TOWNSEND, THE CAUSE OF A SOFT ROT OF TOMATO, AND B. CAROTOVORUS JONES A. B. MASSEY! \Vl'l'll Tlll:EE FIGURES IN THE TEXT INTRODUCTION In the summer of 1918, at Blacksburg, Virginia, there developed a con- siderable amount of a soft rot of tomatoes. This occurre<l in experimental plots which were designated to study the control of septoria leaf blight, and the soft rot of the fruit developed into an important factor. I~ describing these experiments Fromme (2) states: "Practically all of the unsoundness of the fruit was caused by bacterial soft rot, a disease which is exceedingly common and often very destructive in tomato fields in Vir- ginia." Isolations from diseased fruits made by S. A. Wingard (15) proved a bacterium to be the causative agent. Its growth in pure culture resembled that of the group of bacteria which causes soft rots of plants but it could not be readily as!ligned to any of the describe<l species of this group. There has been only casual mention of a bacterial soft rot of tomato in literature, and the distinguishing features of the organisms which might be responsible have not been as sharply defined as is desirable. It was decided, therefore, to un<lcrtake comparative studies of the organism in question together with some of the non-chromogenic soft rot forms. -

Leaf Blotching in Caladium
HORTSCIENCE 44(1):40–43. 2009. (Deng and Harbaugh, 2006; Wilfret, 1986). The presence of leaf spots is controlled by a single locus with two alleles that are inherited Leaf Blotching in Caladium (Araceae) independently from leaf shape but closely linked with the color of the main vein (Deng Is Under Simple Genetic Control and et al., 2008). Another major foliar trait in caladium is Tightly Linked to Vein Color leaf blotching, the occurrence of numerous irregularly shaped color areas between major Zhanao Deng1 and Brent K. Harbaugh veins on leaf blades. Leaf blotches may University of Florida, IFAS, Environmental Horticulture Department, appear singly or coalesce to large areas of Gulf Coast Research and Education Center, 14625 County Road 672, coloration, up to several inches on mature leaves. This pattern of coloration in combi- Wimauma, FL 33598 nation with bright colors has resulted in Additional index words. Caladium ·hortulanum, leaf characteristics, inheritance, breeding, attractive, highly valued and desired cala- ornamental aroids dium cultivars, including Carolyn Whorton and White Christmas. With large pink or Abstract. Cultivated caladiums (Caladium ·hortulanum Birdsey) are valued as important white blotches, ‘Carolyn Whorton’ and pot and landscape plants because of their bright, colorful leaves. Improving leaf ‘White Christmas’ have been the most pop- characteristics or generating new combinations of these characteristics has been one of ular fancy-leaved pink or white cultivars the most important breeding objectives in caladium. A major leaf characteristic in (Bell et al., 1998; Deng et al., 2005). Interest caladium is leaf blotching, the presence of numerous irregularly shaped color areas in incorporating this coloration pattern into between major veins on leaf blades. -

My Green Wet Thumb: Lagenandra
My Green Wet Thumb: Lagenandra By Derek P.S. Tustin Over the years I have found that the average aquarist will go through several different stages. I am by no means a sociologist specializing in the aquari- um hobbyist, but from my own observations I think pretty much everyone goes through some variation of the following; Initial wide-spread interest and associated errors, A focusing of interest into one or two main areas, Competence in an area of interest, Mastery of an area of interest Expansion of interest into new areas while either maintaining the old interest, or focusing entirely on the new area of interest. As an aquatic horticulturist, there are actually very few entry points, or at least entry species, into the hobby. When I started out, I had access to sev- eral excellent aquarium stores with an impressive diversity of aquatic crea- tures, but a very limited selection of aquatic plants. Now, this was back be- fore I joined the Durham Region Aquarium Society (DRAS), so I didn’t have access to mentors or their specialized stock, and it was also before there were so many excellent on-line resources. Most of my initial experience came from the limited genera of plants that were available in local stores; Echinodorus, Cryptocoryne, Anubias and some Aponogeton. (Oh, there were numerous stem plants, but for some reason, I have never been that interested in those, being much more fascinated by rooted plants, and my interest in ponds and suitable plants came much later.) Over the past decade, I have grown the majority of commonly available plants from those genera, and now also have the benefit of being exposed to other skilled hobbyists and resources offered through DRAS. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -

The Exclusively Neotropical Genus Scaphispatha Was Formerly Considered Monospecific. the Type Species
A REVISION OF SCAPHISPATHA (ARACEAE – CALADIEAE) INCLUDING A NEW SPECIES Eduardo Gomes Gonçalves1 ABSTRACT (A revision of Scaphispatha (Araceae – Caladieae) including a new species) The formerly considered monospecific genus Scaphispatha (Araceae – Caladieae) is here revised. Scaphispatha robusta E.G.Gonç, a second species for the genus is newly described from the Cerrado Biome and the transition Cerrado- Amazonia. It differs from S. gracilis Brongn. ex Schott by the much more robust petioles and leaves, primary lateral veins drying clearer than the lamina, lateral secondary veins conspicously more prominent than tertiary veins and for the female spadix with 11-15 rows of flowers visible in side view. A key to separate both species is provided, as well as ink illustrations and general remarks on the genus. Key-words: Scaphispatha, Cerrado, Caladieae, Araceae, geophyte. RESUMO (Revisão de Scaphispatha (Araceae – Caladieae), incluíndo a descrição de uma nova espécie para o gênero) O gênero Scaphispatha (Araceae – Caladieae), até então considerado monoespecífico, é aqui revisado. Scaphispatha robusta E.G.Gonç., uma segunda espécie para o gênero é descrita para o bioma Cerrado e a transição Cerrado-Amazonia. Difere de S. gracilis Brongn. ex Schott pelos pecíolos e folhas muito mais robustas, nervuras laterais mais claras que o limbo quando secas, nervuras laterais secundárias mais proemi- nentes que as terciárias e pela porção feminina do espádice com 11-15 espirais de flores visíveis em vista lateral. Uma chave para separar as espécies, assim como ilustrações em nanquim e aspectos gerais para o gênero são apresentados. Palavras-chave: Scaphispatha, Cerrado, Caladieae, Araceae, geófita. INTRODUCTION was recognized when plants from Pará state The exclusively neotropical genus (Northern Brazil) flowered in cultivation. -

Ornamental Garden Plants of the Guianas, Part 3
; Fig. 170. Solandra longiflora (Solanaceae). 7. Solanum Linnaeus Annual or perennial, armed or unarmed herbs, shrubs, vines or trees. Leaves alternate, simple or compound, sessile or petiolate. Inflorescence an axillary, extra-axillary or terminal raceme, cyme, corymb or panicle. Flowers regular, or sometimes irregular; calyx (4-) 5 (-10)- toothed; corolla rotate, 5 (-6)-lobed. Stamens 5, exserted; anthers united over the style, dehiscing by 2 apical pores. Fruit a 2-celled berry; seeds numerous, reniform. Key to Species 1. Trees or shrubs; stems armed with spines; leaves simple or lobed, not pinnately compound; inflorescence a raceme 1. S. macranthum 1. Vines; stems unarmed; leaves pinnately compound; inflorescence a panicle 2. S. seaforthianum 1. Solanum macranthum Dunal, Solanorum Generumque Affinium Synopsis 43 (1816). AARDAPPELBOOM (Surinam); POTATO TREE. Shrub or tree to 9 m; stems and leaves spiny, pubescent. Leaves simple, toothed or up to 10-lobed, to 40 cm. Inflorescence a 7- to 12-flowered raceme. Corolla 5- or 6-lobed, bluish-purple, to 6.3 cm wide. Range: Brazil. Grown as an ornamental in Surinam (Ostendorf, 1962). 2. Solanum seaforthianum Andrews, Botanists Repository 8(104): t.504 (1808). POTATO CREEPER. Vine to 6 m, with petiole-tendrils; stems and leaves unarmed, glabrous. Leaves pinnately compound with 3-9 leaflets, to 20 cm. Inflorescence a many- flowered panicle. Corolla 5-lobed, blue, purple or pinkish, to 5 cm wide. Range:South America. Grown as an ornamental in Surinam (Ostendorf, 1962). Sterculiaceae Monoecious, dioecious or polygamous trees and shrubs. Leaves alternate, simple to palmately compound, petiolate. Inflorescence an axillary panicle, raceme, cyme or thyrse. -

Caladium Genetics and Breeding: Recent Advances
® Floriculture and Ornamental Biotechnology ©2012 Global Science Books Caladium Genetics and Breeding: Recent Advances Zhanao Deng University of Florida/IFAS, Environmental Horticulture Department, Gulf Coast Research and Education Center, 14625 County Road 672, Wimauma, FL 33598, USA Corresponding author : [email protected] ABSTRACT Caladiums are important ornamental aroids; they are valued for their colourful and variably-shaped leaves. Numerous advances have been made in recent decades in caladium breeding and genetic studies. Techniques have been developed to increase flower production, store pollen, and maintain seed viability. Sources of genetic resistance have been identified for important diseases and pests (such as Fusarium tuber rot, Pythium root rot, bacterial blight, and root-knot nematodes) and abiotic stress factors including chilling injury. Mode of inheritance for important foliar traits has been elucidated through analysis of trait segregation in progeny populations. Caladiums have evolved three alleles at one locus that control colour of leaf main veins (red, white or green) and two co-dominant alleles at an independent locus that determine leaf shapes (fancy, lance, or strap). Gene loci for leaf spotting and blotching are both simply inherited but tightly linked to green veins. In vitro culture and plant regeneration were successful with several types of tissues/organs through somatic embryogenesis and/or organogenesis. Shoot-tip culture has been used to eliminate viral and fungal pathogens and invigorate planting stock; protoplasts isolated from leaf callus regenerated into whole plants; foreign genes from maize or humans have been introduced into caladium through Agrobacterium co-cultivation. Molecular markers, including highly specific and informative SSRs, have been developed and applied to caladium to distinguish cultivars, assess genetic diversity, and analyze genetic relationships. -

A REVIEW Summary Dieffenbachia May Well Be the Most Toxic Genus in the Arace
Journal of Ethnopharmacology, 5 (1982) 293 - 302 293 DIEFFENBACH/A: USES, ABUSES AND TOXIC CONSTITUENTS: A REVIEW JOSEPH ARDITTI and ELOY RODRIGUEZ Department of Developmental and Cell Biology, University of California, Irvine, California 92717 (US.A.) (Received December 28, 1980; accepted June 30, 1981) Summary Dieffenbachia may well be the most toxic genus in the Araceae. Cal cium oxalate crystals, a protein and a nitrogen-free compound have been implicated in the toxicity, but the available evidence is unclear. The plants have also been used as food, medicine, stimulants, and to inflict punishment. Introduction Dieffenbachia is a very popular ornamental plant which belongs to the Araceae. One member of the genus, D. seguine, was cultivated in England before 1759 (Barnes and Fox, 1955). At present the variegated D. picta and its numerous cultivars are most popular. The total number of Dieffenbachia plants in American homes is estimated to be in the millions. The plants can be 60 cm to 3 m tall, and have large spotted and/or variegated (white, yellow, green) leaves that may be 30 - 45 cm long and 15 - 20 cm wide. They grow well indoors and in some areas outdoors. Un fortunately, however, Dieffenbachia may well be the most toxic genus in the Araceae, a family known for its poisonous plants (Fochtman et al., 1969; Pam el, 1911 ). As a result many children (Morton, 1957, 1971 ), adults (O'Leary and Hyattsville, 1964), and pets are poisoned by Dieffenbachia every year (Table 1 ). Ingestion of even a small portion of stem causes a burn ing sensation as well as severe irritation of the mouth, throat and vocal cords (Pohl, 1955). -

Flowers and Foliage Plants
Florida 4-H Horticulture Identification Form Name Contestant No. County Flowers and Foliage Plants Specimen # Common Name Botanical Name African Violet Saintpaulia spp. Aloe Vera Aloe barbadensis Amaryllis Hippeastrum spp. Beach Sunflower Helianthus debilis Begonia Begonia spp. Bird of Paradise Strelitzia reginae Bird’s Nest Fern Asplenium nidus Blanket Flower Gaillardia spp. Caladium Caladium × spp. Calathea Calathea spp. Canna Canna × generalis Celosia Celosia spp. Chinese Evergreen Aglaonema commutatum Chrysanthemum Dendranthema × grandiflorum Coleus Solenostemon scutellarioides Coreopsis, Tickseed Coreopsis spp. Daylily Hemerocallis spp. Dendrobium Orchids Dendrobium spp. Dianthus Dianthus chinensis Dieffenbachia Dieffenbachia spp. Dracaena Marginata Dracaena marginata English Ivy Hedera helix False Heather, Mexican Heather Cuphea hyssopifolia Geranium Pelargonium × hortorum Gerbera Daisy Gerbera jamesonii Globe Amaranth Gomphrena globosa Heart -Leaf Philodendron Philodendron scandens subsp. oxycarium Holly Fern Cyrtomium falcatum Impatiens Impatiens spp. Jade Plant Crassula argentea Lily of the Nile Agapanthus praecox & Agapanthus ssp. orientalis Marigold Tagetes spp. Moth Orchid Phalaenopsis spp. Neanthe Bella Palm Chamaedorea elegans Pentas Pentas lanceolata Peperomia Peperomia spp. Periwinkle Catharanthus roseus Pothos Epipremnum pinnatum ‘Aureum’ Salvia Salvia spp. Sansevieria Sansevieria spp. Spathiphyllum Spathiphyllum spp. Spider Plant Chlorophytum comosum Ti Plant Cordyline terminalis Torenia, Wishbone Flower Torenia fournieri Verbena Verbenia × hybrida Wax Plant Hoya carnosa Weeping Fig Ficus benjamina Zebra Plant Aphelandra squarrosa Zinnia Zinnia elegans ZZ Plant Zamioculcas zamiifolia . -

Gardenergardener®
Theh American A n GARDENERGARDENER® The Magazine of the AAmerican Horticultural Societyy January / February 2016 New Plants for 2016 Broadleaved Evergreens for Small Gardens The Dwarf Tomato Project Grow Your Own Gourmet Mushrooms contents Volume 95, Number 1 . January / February 2016 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS 2016 Seed Exchange catalog now available, upcoming travel destinations, registration open for America in Bloom beautifi cation contest, 70th annual Colonial Williamsburg Garden Symposium in April. 11 AHS MEMBERS MAKING A DIFFERENCE Dale Sievert. 40 HOMEGROWN HARVEST Love those leeks! page 400 42 GARDEN SOLUTIONS Understanding mycorrhizal fungi. BOOK REVIEWS page 18 44 The Seed Garden and Rescuing Eden. Special focus: Wild 12 NEW PLANTS FOR 2016 BY CHARLOTTE GERMANE gardening. From annuals and perennials to shrubs, vines, and vegetables, see which of this year’s introductions are worth trying in your garden. 46 GARDENER’S NOTEBOOK Link discovered between soil fungi and monarch 18 THE DWARF TOMATO PROJECT BY CRAIG LEHOULLIER butterfl y health, stinky A worldwide collaborative breeds diminutive plants that produce seeds trick dung beetles into dispersal role, regular-size, fl avorful tomatoes. Mt. Cuba tickseed trial results, researchers unravel how plants can survive extreme drought, grant for nascent public garden in 24 BEST SMALL BROADLEAVED EVERGREENS Delaware, Lady Bird Johnson Wildfl ower BY ANDREW BUNTING Center selects new president and CEO. These small to mid-size selections make a big impact in modest landscapes. 50 GREEN GARAGE Seed-starting products. 30 WEESIE SMITH BY ALLEN BUSH 52 TRAVELER’S GUIDE TO GARDENS Alabama gardener Weesie Smith championed pagepage 3030 Quarryhill Botanical Garden, California. -
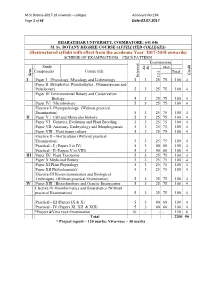
Restructured Syllabi with Effect from the Academic Year 2017-2018 Onwards
M.Sc Botany-2017-18 onwards – colleges Annexure No:19A Page 1 of 43 Date:03.07.2017 BHARATHIAR UNIVERSITY, COIMBATORE: 641 046 M. Sc. BOTANY DEGREE COURSE (AFFILIATED COLLEGES) (Restructured syllabi with effect from the academic Year 2017-2018 onwards) SCHEME OF EXAMINATIONS – CBCS PATTERN Examinations Study Mark Hrs. Dur. Components Course title Total Credit Sem. CIA Ins. hrs/week I Paper I Phycology, Mycology and Lichenology 5 3 25 75 100 4 Paper II Bryophytes, Pteridophytes , Gymnosperms and Paleobotany 5 3 25 75 100 4 Paper III Environmental Botany and Conservation Biology 5 3 25 75 100 4 Paper IV Microbiology 5 3 25 75 100 4 Elective I- Phytopathology (Without practical Examination) 5 3 25 75 100 4 II Paper V Cell and Molecular biology 5 3 25 75 100 4 Paper VI Genetics, Evolution and Plant Breeding 5 3 25 75 100 4 5 Paper VII Anatomy, Embryology and Morphogenesis 5 3 25 75 100 4 Paper VIII Plant tissue culture 5 3 25 75 100 4 Elective II – Horticulture (Without practical Examination) 5 3 25 75 100 4 Practical - I (Papers I to IV) 5 3 40 60 100 4 Practical - II (Papers V to VIII) 5 3 40 60 100 4 III Paper IX Plant Taxonomy 5 3 25 75 100 4 Paper X Medicinal Botany 5 3 25 75 100 4 Paper XI Plant Physiology 5 3 25 75 100 4 Paper XII Phytochemistry 5 3 25 75 100 4 Elective-III Bioinstrumentation and Biological Techniques (Without practical Examination) 5 3 25 75 100 4 IV Paper XIII Biotechnology and Genetic Engineering 5 3 25 75 100 4 Elective IV-Bioinformatics and Biostatistics (Without practical Examination) 5 3 25 75 100 4 Practical - III (Papers IX & X) 5 3 40 60 100 4 Practical - IV (Papers XI, XII & XIII) 5 3 40 60 100 4 *Project &Viva voce Examination 10 - - - 150 6 Total 2250 90 * Project report – 120 marks; Viva-voce – 30 marks M.Sc Botany-2017-18 onwards – colleges Annexure No:19A Page 2 of 43 Date:03.07.2017 Method of implementation and evaluation of Project Based on the strength, students will be allotted to staff members by lot in the first week after reopening the college. -

Natural Hybridization – Recombination – an Ever-Ongoing Process
THAI FOREST BULL., BOT. 47(1): 19–28. 2019. DOI https://doi.org/10.20531/tfb.2019.47.1.05 Natural hybridization – recombination – an ever-ongoing process NIELS JACOBSEN1,* & MARIAN ØRGAARD1 ABSTRACT Exemplified by studies of the SE Asian genusCryptocoryne (Araceae) we provide evidence that: 1) interspecific hybridization is an ever- ongoing process, and introgression and gene exchange takes place whenever physically possible throughout the region; 2) artificial hybridization experiments confirm that wide crosses are possible in a large number of cases; 3) rivers and streams provide numerous, diverse habitats for Cryptocoryne diaspores to settle in; 4) the changes in habitats caused by recurrent glaciations resulting in numerous splitting and merging of populations facilitates hybridization and segregation of subsequent generations; 5) hybridization is a major driving element in speciation; 6) populations are the units and stepping stones in evolution – not the species. KEYWORDS: Araceae, Chromosome numbers, Cryptocoryne, hybridization, evolution. Accepted for publication: 11 January 2019. Published online: 14 February 2019 INTRODUCTION Chiang Khan, Loei Province, hosts a number of morphologically distinct but genetically closely The completion of Cryptocoryne Fisch. ex related hybrids (Idei et al., 2017). Wydler for the Araceae volume for Flora of Thailand stands as a milestone after several years of research in this biological unique and complex genus (Boyce THE GENUS CRYPTOCORYNE et al., 2012). Numerous cultivation experiments, The genus