LD5655.V855 1924.M377.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications
-

Leaf Variegation in Caladium Steudneriifolium (Araceae): a Case of Mimicry?
Evol Ecol (2009) 23:503–512 DOI 10.1007/s10682-008-9248-2 ORIGINAL PAPER Leaf variegation in Caladium steudneriifolium (Araceae): a case of mimicry? Ulf Soltau Æ Stefan Do¨tterl Æ Sigrid Liede-Schumann Received: 27 November 2007 / Accepted: 25 February 2008 / Published online: 6 March 2008 Ó Springer Science+Business Media B.V. 2008 Abstract The leaves of Caladium steudneriifolium (Araceae) of the understorey of a submontane rainforest in the Podocarpus National Park (South East Ecuador, 1,060 m a.s.l.) are plain green or patterned with whitish variegation. Of the 3,413 individual leaves randomly chosen and examined in April 2003, two-thirds were plain green, whereas one third were variegated (i.e., whitish due to absence of chloroplasts). Leaves of both morphs are frequently attacked by mining moth caterpillars. Our BLAST analysis based on Cytochrome-c-Oxidase-subunit-1 sequences suggests that the moth is possibly a member of the Pyraloidea or another microlepidopteran group. It was observed that the variegated leaf zones strongly resemble recent damages caused by mining larvae and therefore may mimic an attack by moth larvae. Infestation was significantly 4–12 times higher for green leaves than for variegated leaves. To test the hypothesis that variegation can be interpreted as mimicry to deter ovipositing moths, we first ruled out the possibility that variegation is a function of canopy density (i.e., that the moths might be attracted or deterred by factors unrelated to the plant). Then plain green leaves were artificially variegated and the number of mining larvae counted after 3 months. -

My Green Wet Thumb: Lagenandra
My Green Wet Thumb: Lagenandra By Derek P.S. Tustin Over the years I have found that the average aquarist will go through several different stages. I am by no means a sociologist specializing in the aquari- um hobbyist, but from my own observations I think pretty much everyone goes through some variation of the following; Initial wide-spread interest and associated errors, A focusing of interest into one or two main areas, Competence in an area of interest, Mastery of an area of interest Expansion of interest into new areas while either maintaining the old interest, or focusing entirely on the new area of interest. As an aquatic horticulturist, there are actually very few entry points, or at least entry species, into the hobby. When I started out, I had access to sev- eral excellent aquarium stores with an impressive diversity of aquatic crea- tures, but a very limited selection of aquatic plants. Now, this was back be- fore I joined the Durham Region Aquarium Society (DRAS), so I didn’t have access to mentors or their specialized stock, and it was also before there were so many excellent on-line resources. Most of my initial experience came from the limited genera of plants that were available in local stores; Echinodorus, Cryptocoryne, Anubias and some Aponogeton. (Oh, there were numerous stem plants, but for some reason, I have never been that interested in those, being much more fascinated by rooted plants, and my interest in ponds and suitable plants came much later.) Over the past decade, I have grown the majority of commonly available plants from those genera, and now also have the benefit of being exposed to other skilled hobbyists and resources offered through DRAS. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -

Caladium Genetics and Breeding: Recent Advances
® Floriculture and Ornamental Biotechnology ©2012 Global Science Books Caladium Genetics and Breeding: Recent Advances Zhanao Deng University of Florida/IFAS, Environmental Horticulture Department, Gulf Coast Research and Education Center, 14625 County Road 672, Wimauma, FL 33598, USA Corresponding author : [email protected] ABSTRACT Caladiums are important ornamental aroids; they are valued for their colourful and variably-shaped leaves. Numerous advances have been made in recent decades in caladium breeding and genetic studies. Techniques have been developed to increase flower production, store pollen, and maintain seed viability. Sources of genetic resistance have been identified for important diseases and pests (such as Fusarium tuber rot, Pythium root rot, bacterial blight, and root-knot nematodes) and abiotic stress factors including chilling injury. Mode of inheritance for important foliar traits has been elucidated through analysis of trait segregation in progeny populations. Caladiums have evolved three alleles at one locus that control colour of leaf main veins (red, white or green) and two co-dominant alleles at an independent locus that determine leaf shapes (fancy, lance, or strap). Gene loci for leaf spotting and blotching are both simply inherited but tightly linked to green veins. In vitro culture and plant regeneration were successful with several types of tissues/organs through somatic embryogenesis and/or organogenesis. Shoot-tip culture has been used to eliminate viral and fungal pathogens and invigorate planting stock; protoplasts isolated from leaf callus regenerated into whole plants; foreign genes from maize or humans have been introduced into caladium through Agrobacterium co-cultivation. Molecular markers, including highly specific and informative SSRs, have been developed and applied to caladium to distinguish cultivars, assess genetic diversity, and analyze genetic relationships. -
Restructured Syllabi with Effect from the Academic Year 2017-2018 Onwards
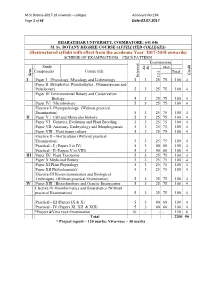
M.Sc Botany-2017-18 onwards – colleges Annexure No:19A Page 1 of 43 Date:03.07.2017 BHARATHIAR UNIVERSITY, COIMBATORE: 641 046 M. Sc. BOTANY DEGREE COURSE (AFFILIATED COLLEGES) (Restructured syllabi with effect from the academic Year 2017-2018 onwards) SCHEME OF EXAMINATIONS – CBCS PATTERN Examinations Study Mark Hrs. Dur. Components Course title Total Credit Sem. CIA Ins. hrs/week I Paper I Phycology, Mycology and Lichenology 5 3 25 75 100 4 Paper II Bryophytes, Pteridophytes , Gymnosperms and Paleobotany 5 3 25 75 100 4 Paper III Environmental Botany and Conservation Biology 5 3 25 75 100 4 Paper IV Microbiology 5 3 25 75 100 4 Elective I- Phytopathology (Without practical Examination) 5 3 25 75 100 4 II Paper V Cell and Molecular biology 5 3 25 75 100 4 Paper VI Genetics, Evolution and Plant Breeding 5 3 25 75 100 4 5 Paper VII Anatomy, Embryology and Morphogenesis 5 3 25 75 100 4 Paper VIII Plant tissue culture 5 3 25 75 100 4 Elective II – Horticulture (Without practical Examination) 5 3 25 75 100 4 Practical - I (Papers I to IV) 5 3 40 60 100 4 Practical - II (Papers V to VIII) 5 3 40 60 100 4 III Paper IX Plant Taxonomy 5 3 25 75 100 4 Paper X Medicinal Botany 5 3 25 75 100 4 Paper XI Plant Physiology 5 3 25 75 100 4 Paper XII Phytochemistry 5 3 25 75 100 4 Elective-III Bioinstrumentation and Biological Techniques (Without practical Examination) 5 3 25 75 100 4 IV Paper XIII Biotechnology and Genetic Engineering 5 3 25 75 100 4 Elective IV-Bioinformatics and Biostatistics (Without practical Examination) 5 3 25 75 100 4 Practical - III (Papers IX & X) 5 3 40 60 100 4 Practical - IV (Papers XI, XII & XIII) 5 3 40 60 100 4 *Project &Viva voce Examination 10 - - - 150 6 Total 2250 90 * Project report – 120 marks; Viva-voce – 30 marks M.Sc Botany-2017-18 onwards – colleges Annexure No:19A Page 2 of 43 Date:03.07.2017 Method of implementation and evaluation of Project Based on the strength, students will be allotted to staff members by lot in the first week after reopening the college. -

Natural Hybridization – Recombination – an Ever-Ongoing Process
THAI FOREST BULL., BOT. 47(1): 19–28. 2019. DOI https://doi.org/10.20531/tfb.2019.47.1.05 Natural hybridization – recombination – an ever-ongoing process NIELS JACOBSEN1,* & MARIAN ØRGAARD1 ABSTRACT Exemplified by studies of the SE Asian genusCryptocoryne (Araceae) we provide evidence that: 1) interspecific hybridization is an ever- ongoing process, and introgression and gene exchange takes place whenever physically possible throughout the region; 2) artificial hybridization experiments confirm that wide crosses are possible in a large number of cases; 3) rivers and streams provide numerous, diverse habitats for Cryptocoryne diaspores to settle in; 4) the changes in habitats caused by recurrent glaciations resulting in numerous splitting and merging of populations facilitates hybridization and segregation of subsequent generations; 5) hybridization is a major driving element in speciation; 6) populations are the units and stepping stones in evolution – not the species. KEYWORDS: Araceae, Chromosome numbers, Cryptocoryne, hybridization, evolution. Accepted for publication: 11 January 2019. Published online: 14 February 2019 INTRODUCTION Chiang Khan, Loei Province, hosts a number of morphologically distinct but genetically closely The completion of Cryptocoryne Fisch. ex related hybrids (Idei et al., 2017). Wydler for the Araceae volume for Flora of Thailand stands as a milestone after several years of research in this biological unique and complex genus (Boyce THE GENUS CRYPTOCORYNE et al., 2012). Numerous cultivation experiments, The genus -

History and Current Status of Systematic Research with Araceae
HISTORY AND CURRENT STATUS OF SYSTEMATIC RESEARCH WITH ARACEAE Thomas B. Croat Missouri Botanical Garden P. O. Box 299 St. Louis, MO 63166 U.S.A. Note: This paper, originally published in Aroideana Vol. 21, pp. 26–145 in 1998, is periodically updated onto the IAS web page with current additions. Any mistakes, proposed changes, or new publications that deal with the systematics of Araceae should be brought to my attention. Mail to me at the address listed above, or e-mail me at [email protected]. Last revised November 2004 INTRODUCTION The history of systematic work with Araceae has been previously covered by Nicolson (1987b), and was the subject of a chapter in the Genera of Araceae by Mayo, Bogner & Boyce (1997) and in Curtis's Botanical Magazine new series (Mayo et al., 1995). In addition to covering many of the principal players in the field of aroid research, Nicolson's paper dealt with the evolution of family concepts and gave a comparison of the then current modern systems of classification. The papers by Mayo, Bogner and Boyce were more comprehensive in scope than that of Nicolson, but still did not cover in great detail many of the participants in Araceae research. In contrast, this paper will cover all systematic and floristic work that deals with Araceae, which is known to me. It will not, in general, deal with agronomic papers on Araceae such as the rich literature on taro and its cultivation, nor will it deal with smaller papers of a technical nature or those dealing with pollination biology. -

A Review of the Aroid Tribe Caladieae with the Description of Three New Species of Caladium and Seven New Species of Syngonium (Araceae)
A Review of the Aroid Tribe Caladieae with the Description of Three New Species of Caladium and Seven New Species of Syngonium (Araceae) Thomas B. Croat,1 Xavier Delannay,2 Orlando O. Ortiz,3 and Pedro Diaz Jim´enez4 1 P. A. Schulze Curator, Missouri Botanical Garden, 4344 Shaw Blvd., St. Louis, Missouri 63110, U.S.A. [email protected] 2 Volunteer Research Associate, Missouri Botanical Garden, 4344 Shaw Blvd., St. Louis, Missouri 63110, U.S.A. 3 Herbario PMA & Programa de Maestr´ıa en Ciencias Biol´ogicas, Universidad de Panam´a, Estafeta Universitaria, Panama City, Panama. 4 Centro de Investigaciones Tropicales, Universidad Veracruzana, Xalapa, Veracruz, Mexico. ABSTRACT. A review of the aroid tribe Caladieae is that summarized our knowledge of the group, described presented, and three new species of Caladium Vent. new species, and discovered important distinctions and seven new species of Syngonium Schott are de- between Caladium and Xanthosoma, markedly that scribed and illustrated. Two species, C. picturatum K. differences occur in the display of their pollen. Pollen Koch & C. D. Bouch´e and C. steudnerifolium Engl., grains are borne in tetrads in Xanthosoma and solitarily previously considered to be synonymous with C. bicolor in Caladium. Madison (1981) provided a key to the six (Aiton) Vent., are fully redescribed. New species of genera considered to be Caladieae at that time, namely Caladium are C. cortesae Croat & E. G. Gonç., C. Aphyllarum S. Moore, Caladium, Chlorospatha, Jasa- palaciosii Croat & L. P. Hannon, and C. stevensonii rum, Scaphispatha, and Xanthosoma. Aphyllarum has Croat & Delannay. New species of Syngonium are S. -

Phylogenetic Analyses of Cretaceous Fossils Related to Chloranthaceae and Their Evolutionary Implications
UC Davis UC Davis Previously Published Works Title Phylogenetic Analyses of Cretaceous Fossils Related to Chloranthaceae and their Evolutionary Implications Permalink https://escholarship.org/uc/item/0d58r5r0 Journal Botanical Review, 84(2) ISSN 0006-8101 Authors Doyle, JA Endress, PK Publication Date 2018-06-01 DOI 10.1007/s12229-018-9197-6 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Phylogenetic Analyses of Cretaceous Fossils Related to Chloranthaceae and their Evolutionary Implications James A. Doyle & Peter K. Endress The Botanical Review ISSN 0006-8101 Volume 84 Number 2 Bot. Rev. (2018) 84:156-202 DOI 10.1007/s12229-018-9197-6 1 23 Your article is protected by copyright and all rights are held exclusively by The New York Botanical Garden. This e-offprint is for personal use only and shall not be self- archived in electronic repositories. If you wish to self-archive your article, please use the accepted manuscript version for posting on your own website. You may further deposit the accepted manuscript version in any repository, provided it is only made publicly available 12 months after official publication or later and provided acknowledgement is given to the original source of publication and a link is inserted to the published article on Springer's website. The link must be accompanied by the following text: "The final publication is available at link.springer.com”. 1 23 Author's personal copy Bot. Rev. (2018) 84:156–202 https://doi.org/10.1007/s12229-018-9197-6 Phylogenetic Analyses of Cretaceous Fossils Related to Chloranthaceae and their Evolutionary Implications James A. -

The Status of Cryptocoryne Annamica (Araceae: Aroideae: Cryptocoryneae) in Vietnam
Gardens’ Bulletin Singapore 66(1): 67–72. 2014 67 The status of Cryptocoryne annamica (Araceae: Aroideae: Cryptocoryneae) in Vietnam V.D. Nguyen1,*, H.Q. Bui1 & Josef Bogner2 1 Institute of Ecology and Biological Resources – Vietnam Academy of Sciences and Technology, 18 Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam *[email protected] 2Augsburger Str. 43a, D-86368 Gersthofen, Germany ABSTRACT. Cryptocoryne annamica Serebryanyi, an endemic species from Gia Lai province in Vietnam, was first described in 1991. Until recently only two collections were known but a new collection has now been made in the Kon Ka King National Park, Gia Lai province. The species description is elaborated and notes on its biology, distribution, ecology and cultivation are given. A proposed IUCN conservation assessment is given. A key to the Cryptocoryne species of Vietnam is included. Keywords. Conservation, cultivation, distribution, ecology, taxonomy Introduction Gagnepain (1942) recognised four species of the genus Cryptocoryne Fisch. ex Wydler for Vietnam, namely C. ciliata (Roxb.) Fisch. ex Wydler, C. retrospiralis (Roxb.) Kunth, C. tonkinensis Gagnep. and C. balansae Gagnep. Jacobsen (1991) reduced two of Gagnepain’s species to varieties of C. crispatula Engl.: C. crispatula var. tonkinensis (Gagnep.) N.Jacobsen and C. crispatula var. balansae (Gagnep.) N.Jacobsen. Cryptocoryne retrospiralis has not subsequently been confirmed as occurring in Vietnam. Since then Cryptocoryne annamica Serebryanyi (1991) and C. vietnamensis Hertel & Mühlberg (1994) have been described. Therefore, there are four species of Cryptocoryne recorded for Vietnam, one of them with three varieties: C. crispatula var. tonkinensis, C. crispatula var. yunnanensis (H.Li) H.Li & N.Jacobsen, C. -

Typification of Arisarum Proboscideum
53 (4) • November 2004: 1047–1048 Galán de Mera & Castroviejo • Typification in Araceae Typification of two Linnaean names in Araceae: Arum proboscideum L. and Arum muscivorum L. f. Antonio Galán de Mera1 & Santiago Castroviejo2 1 Departamento de Ciencias Ambientales y Recursos Naturales, Laboratorio de Botánica, Universidad San Pablo-CEU, P.O. Box 67, E-28660 Boadilla del Monte, Madrid, Spain. [email protected] (author for corre- spondence) 2 Real Jardín Botánico, CSIC, Plaza de Murillo 2, E-28014 Madrid, Spain. [email protected] As part of a study of Iberian and Balearic Araceae, two Linnaean names, Arum proboscideum L. and A. mus- civorum L. f., are lecto- and neotypified, respectively. KEYWORDS: Araceae, Arum muscivorum L. f., A. proboscideum L., lectotypification, neotypification. Typification of Linnaean plant names involves con- Part. 2. 61. Tab. 50”] as Boccone writes (“Habbiamo sideration of Linnaeus’s original sources of information havuto questa Pianta dalle memorie del Padre Maestro (collections and illustrations) in association with each Bareliero. Varia dalla nostra Siciliana Potamogeiti folio protologue (Cannon & al., 1983; Jarvis & Cafferty, per la nota della Proboscide, e delle Foglie.”). Barrelier 2003). In the indigenous Araceae of the Iberian worked before Boccone, but his publication only Peninsula and Balearic Islands there are some Linnaean appeared posthumously (see also Savi, 1816). Linnaeus names, Arum arisarum L., A. maculatum L. and A. pic- did not, apparently, see any material of Arum pro- tum L. f., whose types are established (Savage, 1945; boscideum but, like Barrelier (p. 20), recorded this Boyce, 1993). However, the nomenclatural and taxo- species from the Apennines. -

Pollinators and Visitors of Aroid Inflorescences
Pollinators and Visitors of Aroid Inflorescences Marc Gibernau Laboratoire d’E´volution & Diversite´Biologique Universite´de Toulouse III 118 Route de Narbonne, Baˆt. IV R 3—B 2 31062 Toulouse Cedex 4 France e-mail: [email protected] ABSTRACT view of this subject, as Thomas Croat (2000) did in his history and current status Data on aroid pollinators was first sum- of systematic research with Araceae, but to marized by Grayum (1984) who docu- give, in the first place, a statement of the mented 35 genera and about 90 species. A subject and to develop some remarks on second summary was published in 1997 in aroid pollination. The Genera of Araceae (Mayo et al., 1997) with 38 genera and less than 100 species listed including data from Grayum (1986, RESULTS 1990). This paper brings the reference list Summarizing data from Grayum (1984, up to date since 1997, documenting the 1990) and Mayo et al. (1997), and includ- pollinators of 49 genera and about 125 ing omitted and new publications, the pol- species. These numbers are still very low in comparison with the diversity of the Ar- linators of 49 genera and about 125 spe- aceae family which contains 105 genera cies are documented in Table 1. These and about 3,300 species. Some questions numbers are still very low in comparison on aroid pollination are developed in the with the diversity of the Araceae family discussion. which contains 105 genera and about 3,300 species (Mayo et al., 1997). Thus, KEY WORDS pollinators are cited for only 47% of the genera.