First Report of Amynthas Carnosus (Goto & Hatai
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Size Variation and Geographical Distribution of the Luminous Earthworm Pontodrilus Litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan
A peer-reviewed open-access journal ZooKeys 862: 23–43 (2019) Size variation and distribution of Pontodrilus litoralis 23 doi: 10.3897/zookeys.862.35727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan Teerapong Seesamut1,2,4, Parin Jirapatrasilp2, Ratmanee Chanabun3, Yuichi Oba4, Somsak Panha2 1 Biological Sciences Program, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 2 Ani- mal Systematics Research Unit, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Program in Animal Science, Faculty of Agriculture Technology, Sakon Nakhon Rajabhat University, Sakon Nakhon 47000, Thailand 4 Department of Environmental Biology, Chubu University, Kasugai 487-8501, Japan Corresponding authors: Somsak Panha ([email protected]), Yuichi Oba ([email protected]) Academic editor: Samuel James | Received 24 April 2019 | Accepted 13 June 2019 | Published 9 July 2019 http://zoobank.org/663444CA-70E2-4533-895A-BF0698461CDF Citation: Seesamut T, Jirapatrasilp P, Chanabun R, Oba Y, Panha S (2019) Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. ZooKeys 862: 23–42. https://doi.org/10.3897/zookeys.862.35727 Abstract The luminous earthworm Pontodrilus litoralis (Grube, 1855) occurs in a very wide range of subtropical and tropical coastal areas. Morphometrics on size variation (number of segments, body length and diameter) and genetic analysis using the mitochondrial cytochrome c oxidase subunit 1 (COI) gene sequence were conducted on 14 populations of P. -
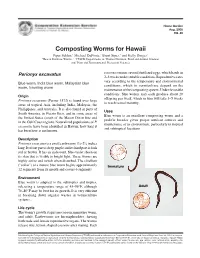
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

The Giant Palouse Earthworm (Driloleirus Americanus)
PETITION TO LIST The Giant Palouse Earthworm (Driloleirus americanus) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT June 30, 2009 Friends of the Clearwater Center for Biological Diversity Palouse Audubon Palouse Prairie Foundation Palouse Group of the Sierra Club 1 June 30, 2009 Ken Salazar, Secretary of the Interior Robyn Thorson, Regional Director U.S. Department of the Interior U.S. Fish & Wildlife Service 1849 C Street N.W. Pacific Region Washington, DC 20240 911 NE 11th Ave Portland, Oregon Dear Secretary Salazar, Friends of the Clearwater, Center for Biological Diversity, Palouse Prairie Foundation, Palouse Audubon, Palouse Group of the Sierra Club and Steve Paulson formally petition to list the Giant Palouse Earthworm (Driloleirus americanus) as a threatened or endangered species pursuant to the Endangered Species Act (”ESA”), 16 U.S.C. §1531 et seq. This petition is filed under 5 U.S.C. 553(e) and 50 CFR 424.14 (1990), which grant interested parties the right to petition for issuance of a rule from the Secretary of Interior. Petitioners also request that critical habitat be designated for the Giant Palouse Earthworm concurrent with the listing, pursuant to 50 CFR 424.12, and pursuant to the Administrative Procedures Act (5 U.S.C. 553). The Giant Palouse Earthworm (D. americanus) is found only in the Columbia River Drainages of eastern Washington and Northern Idaho. Only four positive collections of this species have been made within the last 110 years, despite the fact that the earthworm was historically considered “very abundant” (Smith 1897). The four collections include one between Moscow, Idaho and Pullman, Washington, one near Moscow Mountain, Idaho, one at a prairie remnant called Smoot Hill and a fourth specimen near Ellensberg, Washington (Fender and McKey- Fender, 1990, James 2000, Sánchez de León and Johnson-Maynard, 2008). -

Checklist of New Zealand Earthworms Updated from Lee (1959) by R.J
Checklist of New Zealand Earthworms updated from Lee (1959) by R.J. Blakemore August, 2006 COE fellow, Soil Ecology Group, Yokohama National Univeristy, Japan. Summary This review is based on Blakemore (2004) that updated the work completed over 40 years earlier by Lee (1959) as modified by Blakemore in Lee et al. , (2000) and in Glasby et al. (2007/8) based on the information presented at the "Species 2000" meeting held Jan. 2000 at Te Papa Museum in Wellington, New Zealand. In the current checklist Acanthodrilidae, Octochaetidae and Megascolecidae sensu Blakemore (2000b) are all given separate family status. Whereas Lee (1959) listed approximately 193 species, the current list has about 199 taxa. Because many of the natives have few reports, or are based on only a few specimens, approximately 77 are listed as "threatened" or "endangered" in Dept. Conservation threatened species list (see www.doc.govt.nz/Conservation/001~Plants-and-Animals/006~Threatened-species/Terrestrial-invertebrate-(part-one).asp April, 2005) and three species are detailed in McGuinness (2001). Further studies such as those of Springett & Grey (1998) are required. Currently I seek funding to complete my database into an interactive guide to species, to use to conduct surveys in New Zealand. Some of the changes in Blakemore (2004) from Lee (1959) are: • Microscolex macquariensis (Beddard, 1896) is removed from the list because it is known only from Macquarie Island, which is now claimed as Australian territory (see Blakemore, 2000b). • Megascolides orthostichon (Schmarda, 1861) is removed from the fauna as Fletcher (1886: 524) reported that “on the authority of Captain Hutton” this species was not from New Zealand and may be from Mt Wellington in Tasmania (see Blakemore, 2000b). -

Ecological Monitoring at Rare, 2020
Ecological Monitoring 2020 rare Charitable Research Reserve Prepared by: Jordan Wrobel Jenna Quinn 1 Acknowledgements Many thanks to Colleges and Institutes Canada (CICan) Career-Launcher Internships, funded by Natural Resource’s Canada’s Green Jobs- Science and Technology Internship Program, and Employment Ontario for providing essential funding for ecological monitoring at rare; without their support, this monitoring program and report would not have been possible. I would also like to thank rare staff for assistance with monitoring and support of intellectual and professional growth. Thank you to Caroline Reisiger and Sarah Cui for their much- appreciated assistance with fieldwork and to Dr. Justin Gaudon for your support with the statistical analyses. To rare’s committed volunteers: Jacqueline Haynes, Miriam Bauman, Emma Wegener, Hilary Irving, Bethany Wakefield, and Logan Mercier; thank you so much for your support with monitoring, these programs would not be as successful without you. I would like to thank all advocates of rare Charitable Research Reserve for helping to support rare’s vision and activities. The rare Charitable Research Reserve acknowledges and is grateful to all of the original stewards of the land in which rare resides, within the Haldimand Tract, spanning six miles on either side of the Grand River from source to mouth. Understanding that this land has been rich in diverse Indigenous presence since time immemorial, there are several Indigenous Nations that we would like to mention. We would like to honor and respect the sovereignty of both First Nations in our area: the Haudenosaunee Peoples of Six Nations of the Grand River and the Anishinaabe Peoples of Mississaugas of the New Credit First Nation. -

Amynthas) in the Northeast United States
Invertebrate Biology x(x): 1–14. © 2016 The Authors. Invertebrate Biology published by Wiley Periodicals, Inc. on behalf of American Microscopical Society. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made. DOI: 10.1111/ivb.12145 Phylogeographic analysis of invasive Asian earthworms (Amynthas) in the northeast United States Nancy Schult, Kelly Pittenger, Sam Davalos, and Damhnait McHugha Department of Biology, Colgate University, Hamilton, New York 13346, USA Abstract. Phylogeographic studies are useful in reconstructing the history of species inva- sions, and in some instances can elucidate cryptic diversity of invading taxa. This can help in predicting or managing the spread of invasive species. Among terrestrial invasive species in North America, earthworms can have profound ecological effects. We are familiar with the centuries-old invasions of European earthworms (Lumbricidae) and their impacts on nutrient cycling in soils. More recent invasions by Asian earthworms of the family Megascolecidae are less fully understood. We used data for two mitochondrial gene frag- ments, cytochrome oxidase I (COI) and 16S rRNA, to examine the relationships among populations of Asian earthworms in the megascolecid genus Amynthas in the northeast Uni- ted States. Recent reports have indicated that one species in particular, Amynthas agrestis, is having detrimental effects in mixed forest ecosystems, and we were interested in under- standing the invasion history for this species. We were surprised to discover three divergent mitochondrial lineages of Amynthas occurring sympatrically in upstate New York. -

Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems
Colby College Digital Commons @ Colby Honors Theses Student Research 2019 Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems Ella L. Maddi Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Environmental Sciences Commons, and the Soil Science Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Maddi, Ella L., "Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems" (2019). Honors Theses. Paper 965. https://digitalcommons.colby.edu/honorstheses/965 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented to the Faculty of the Department of Biology at Colby College in partial fulfillment of the requirements for the Degree of Bachelor of Arts with Honors By Ella Maddi Waterville, ME May 20, 2019 Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented -

An Integrative Taxonomic Approach to the Identification of Three New New Zealand Endemic Earthworm Species (Acanthodrilidae, Octochaetidae: Oligochaeta)
Zootaxa 2994: 21–32 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) An integrative taxonomic approach to the identification of three new New Zealand endemic earthworm species (Acanthodrilidae, Octochaetidae: Oligochaeta) STEPHANE BOYER1,3, ROBERT J. BLAKEMORE2 & STEVE D. WRATTEN1 1Bio-Protection Research Centre, Lincoln University, New Zealand 2National Museum of Science and Nature in Tokyo, Japan 3Corresponding author. E-mail: [email protected] Abstract This work adds three new species to the ca. 200 currently known from New Zealand. In Acanthodrilidae is Maoridrilus felix and in Octochaetidae are Deinodrilus gorgon and Octochaetus kenleei. All three are endemics that often have restrict- ed ranges; however, little is yet known of their distribution, ecology nor conservation status. DNA barcoding was conduct- ed, which is the first time that New Zealand endemic holotypes have been so characterized. The barcoding region COI (cytochrome c oxidase subunit 1) as well as the 16S rDNA region were sequenced using tissue from the holotype specimen to provide indisputable uniqueness of the species. These DNA sequences are publically available on GenBank to allow accurate cross checking to verify the identification of other specimens or even to identify specimens on the basis of their DNA sequences alone. Based on their 16S rDNA sequences, the position of the three newly described species in the phy- logeny of New Zealand earthworms was discussed. The description of new species using this approach is encouraged, to provide a user-friendly identification tool for ecologists studying diverse endemic faunas of poorly known earthworm species. -

Phylogeny of the Megascolecidae and Crassiclitellata (Annelida
Phylogeny of the Megascolecidae and Crassiclitellata (Annelida, Oligochaeta): combined versus partitioned analysis using nuclear (28S) and mitochondrial (12S, 16S) rDNA Barrie G. M. JAMIESON Department of Zoology and Entomology, University of Queensland, Brisbane 4072, Queensland (Australia) [email protected] Simon TILLIER Annie TILLIER Département Systématique et Évolution et Service de Systématique moléculaire, Muséum national d’Histoire naturelle, 43 rue Cuvier, F-75231 Paris cedex 05 (France) Jean-Lou JUSTINE Laboratoire de Biologie parasitaire, Protistologie, Helminthologie, Département Systématique et Évolution, Muséum national d’Histoire naturelle, 61 rue Buffon, F-75321 Paris cedex 05 (France) present address: UR “Connaissance des Faunes et Flores Marines Tropicales”, Centre IRD de Nouméa, B.P. A5, 98848 Nouméa cedex (Nouvelle-Calédonie) Edmund LING Department of Zoology and Entomology, University of Queensland, Brisbane 4072, Queensland (Australia) Sam JAMES Department of Life Sciences, Maharishi University of Management, Fairfield, Iowa 52557 (USA) Keith MCDONALD Queensland Parks and Wildlife Service, PO Box 834, Atherton 4883, Queensland (Australia) Andrew F. HUGALL Department of Zoology and Entomology, University of Queensland, Brisbane 4072, Queensland (Australia) ZOOSYSTEMA • 2002 • 24 (4) © Publications Scientifiques du Muséum national d’Histoire naturelle, Paris. www.zoosystema.com 707 Jamieson B. G. M. et al. Jamieson B. G. M., Tillier S., Tillier A., Justine J.-L., Ling E., James S., McDonald K. & Hugall A. F. 2002. — Phylogeny of the Megascolecidae and Crassiclitellata (Annelida, Oligochaeta): combined versus partitioned analysis using nuclear (28S) and mitochondrial (12S, 16S) rDNA. Zoosystema 24 (4) : 707-734. ABSTRACT Analysis of megascolecoid oligochaete (earthworms and allies) nuclear 28S rDNA and mitochondrial 12S and 16S rDNA using parsimony and likeli- hood, partition support and likelihood ratio tests, indicates that all higher, suprageneric, classifications within the Megascolecidae are incompatible with the molecular data. -

External Morphological Comparison, Taxonomic Revision and Molecular Differentiation of the Four Economically Important Species of Earthworm in Thailand
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 10–607/SRZ/2011/13–4–553–558 http://www.fspublishers.org Full Length Article External Morphological Comparison, Taxonomic Revision and Molecular Differentiation of the Four Economically Important Species of Earthworm in Thailand WIRIYA LOONGYAI1, PHUWADOL BANGRAK† AND SOMCHAI CHANTSAVANG Department of Animal Science, Faculty of Agriculture, Kasetsart University, Bangkok 10900 Thailand †School of Science, Walailak University, Thasala, Nakhon Si Thammarat 80160 Thailand 1Corresponding author’s e-mail: [email protected]; [email protected] ABSTRACT Four economically important species of earthworm were cultured and the external and internal characters of adult clitellate earthworms were studied. Partial sequences for ribosomal 16S rDNA and subunit one for mitochondrial cytochrome c oxidase (COI) of four earthworm species were obtained. The result of sequence analysis combined with taxonomic characters could distinguish the different species of earthworm. Morphology and nucleotide sequence of two genes for the red worm (Pheretima peguana) were distinct from Eudrilus eugeniae but were similar to the blue worm (Perionyx excavatus) and Lao worm (P. excavates) and therefore, it was classified as a new species, Perionyx sp. 1. Moreover, Eudrilus eugeniae was evidently defined as the same genus and species. Interestingly, the blue worm and Lao worm were morphologically similar to Perionyx sp. However, the molecular data of 16S rDNA could not differentiate in taxa of those two species. COI nucleotide sequence analyses showed the presence of divergent lineages between two species, suggesting the blue worm and Lao worm could be described as Perionyx sp. 2 and Perionyx sp. -

Giant Gippsland Earthworm Surveys at Three Sites Within the Lang Lang
Giant Gippsland Earthworm survey at a site of a proposed rezoning, Jumbunna Rd, Korumburra February 2012 PREPAREDPREPARED FOR: FOR: REPORT AUTHOR: PREPARED FOR: Company Name Goes Here Dr. Beverley Van Praagh Company Name Goes Here Second Line of Text Here REPORT AUTHOR: Second Line of Text Here REPORT AUTHOR: BeveridgeThird Line Williams of Text & CoHere Pty Ltd Dr. Beverley Van Praagh 52A ThirdBair Street Line of Text Here Dr. Beverley Van Praagh PO Box 161 Leongatha Vic 3953 INVERT-ECO 1 Giant Gippsland Earthworm survey at Jumbunna Rd, Korumburra REPORT - Giant Gippsland Earthworm survey of site of a proposed rezoning, Jumbunna Rd, Korumburra Report by Beverley Van Praagh INVERT-ECO ABN 96 817 328 909 25 Jacaranda Place Craigieburn, Victoria 3064 Tel/Fax: 03 9305 5154 Mobile: 0402 572 443 On Behalf Of Beveridge Williams & Co Pty Ltd 52A Bair Street PO Box 161 Leongatha Vic 3953 Final Version February 2012 ACKNOWLEDGEMENTS The author thanks the following people for their contribution to the project, Neil Breeden (Beveridge Williams) for project and site information Mr Winterhalter and Mr Cellante (property owners) for site access Matt Killin for assistance in the field Cover Photo: Megascolides australis and subject land© INVERT-ECO Abbreviations DSE: Department of Sustainability and Environment EPBC Act: Environment Protection and Biodiversity Conservation Act 1999 FFG Act: Flora and Fauna Guarantee Act 1988 Copyright ©INVERT-ECO This document is subject to copyright and may only be used for the purposes for which it was commissioned. Disclaimer Although due diligence was used by INVERT-ECO in the preparation of this report, INVERT-ECO takes no liability for any damages or loss incurred as a result of reliance placed upon this report and its contents. -

The Earthworm Genus Pheretima, Kinberg, 1866, in Louisiana (Oligochaeta: Megascolecidae)." (1969)
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 The aE rthworm Genus Pheretima, Kinberg, 1866, in Louisiana (Oligochaeta: Megascolecidae). Richard Elvin Tandy Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Tandy, Richard Elvin, "The Earthworm Genus Pheretima, Kinberg, 1866, in Louisiana (Oligochaeta: Megascolecidae)." (1969). LSU Historical Dissertations and Theses. 1566. https://digitalcommons.lsu.edu/gradschool_disstheses/1566 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 69-17,130 TANDY, Richard Elvin, 1937- THE EARTHWORM GENUS PHERETIMA KINBERG, 1866, IN LOUISIANA (OLIGOCHAETA: MEGASCOLECIDAE). Louisiana State University and Agricultural and Mechanical College, Ph.D., 1969 Zoology University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. The Earthworm Genus Pheretima Kinberg, 1856, In Louisiana (01igochaeta:M egascolecidae) A D issertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillm ent of the requirements for the degree of Doctor of Philosophy in The Department of Zoology and Physiology by Richard Elvin Tandy B.A., Anderson College, 1960 M.S., Louisiana Polytechnic Institute, 1963 January, 1969 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGMENT Sincere appreciation is expressed to Dr.