Giant Gippsland Earthworm Megascolides Australis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Invasive Earthworm Lumbricus Terrestris on The
The Effect of Invasive Earthworm Lumbricus terrestris on the Distribution of Nitrogen in Soil Profile Sarah Adelson, Christine Doman, Gillian Golembiewski, Luke Middleton University of Michigan Biological Station, Spring 2009 Abstract The purpose of this study was to determine if Lumbricus terrestris, an invasive earthworm in Northern Michigan, is redistributing nitrogen from the organic soil layer to the deeper, mineral soil layer. L. terrestris burrow 2 meters vertically into the ground and emerge to feed on freshly fallen leaf litter. The study included collecting of L. terrestris in 16 0.5 m square plots by method of electro-shock. Soil cores from a depth of 0-5 and 30-40 cm as well as leaf litter were taken from each plot to determine nitrogen content and nitrogen isotope ratios. Data analysis resulted in no significance between plots with earthworms and without earthworms in both nitrogen, N, isotope ratios and N content. Plots with L. terrestris showed no difference between the organic and mineral soil layer. This result suggests that L. terrestris are homogenizing soil layers. However, smaller than ideal sample sizes limit interpretive capacity of the results. Further research needs to be completed to confirm these perceived trends. The analysis of nitrogen isotope ratios suggest that there is another source of 15N other than leaf litter and L. terrestris that is contributing to soil composition and therefore the contribution of each was not conclusively determined. Introduction Invasion of an exotic species into an ecosystem is one of the leading threats to biologically diverse ecosystems throughout the world. Exotic species are initially introduced as a solution for food, farming, aesthetic purposes, or even accidentally. -

Earthworm Annelid Dissection External Anatomy
Name: Section: Earthworm Annelid Dissection External Anatomy Examine your preserved earthworm and determine the anterior and posterior ends in addition to its dorsal and ventral sides. The ventral side will be slightly flattened and will have two openings types of opening. Locate the worm's mouth and anus. Near the mouth will be a fleshy protruberance called the prostomium. The prostomium is located on the dorsal surface of the worm. Note the swelling of the earthworm near its anterior side - this is the clitellum. Label the clitellum on the drawing. The clitellum is active in the formation of an egg capsule, or cocoon. The openings toward the anterior of the worm are the male genital pores or sperm ducts. There are two pairs. The first pair is anterior to the second pair. The second pair is called the genital setae. They are tiny and are located just above the clitellum. They may be too small to see but may be visualized using a dissecting microscope. Sperm are produced in the testes and pass out through these pores. Just above the first pair of sperm ducts are the female genital pores. Again, these may be too small to see without a dissecting microscope. Eggs are produced in the ovaries and pass out of the body through these pores. Locate the dark line that runs down the dorsal side of the worm, this is the dorsal blood vessel. The ventral blood vessel can be seen on the underside of the worm, though it is usually not as dark. Internal Anatomy 1. Place the specimen in the dissecting pan DORSAL side up. -

Chapter XXV —Class Oligochaeta
Chapter XXV —Class Oligochaeta (Aquatic Worms)- Phylum Annelida Oligochaetes are common in most freshwater habitats, but they are often ignored by freshwater biologists because they are thought to be extraordinarily difficult to identify. The extensive taxo- nomic work done since 1960 by Brinkhurst and others, however, has enabled routine identifica- tion of most of our freshwater oligochaetes from simple whole mounts. Some aquatic worms closely resemble terrestrial earthworms while others can be much narrower or thread-like. Many aquatic worms can tolerate low dissolved oxygen and may be found in large numbers in organi- cally polluted habitats. Aquatic worms can be distinguished by: (Peckarsky et al., 1990) • Body colour may be red, tan, brown or black. • Cylindrical, thin (some are very thin), segmented body may be upto 5 inches. • May have short bristles or hairs (setae) that help with movement (usually not visible). • Moves by stretching and pulling its body along in a worm-like fashion. Four families in the orders Tubificida and Lumbriculida are common in freshwater in northeastern North America: the Tubificidae, Naididae, Lumbriculidae, and Enchytraeidae. In addition, fresh- water biologists sometimes encounter lumbricine oligochaetes (order Lumbricina; the familiar earthworms), haplotaxid oligochaetes (order Haplotaxida; rare inhabitants of groundwater), Aeolosoma (class Aphanoneura; small worms once classified with the oligochaetes), and Manayunkia speciosa (class Polychaeta) in waters of northeastern North America. (Peckarsky et al., 1990). The two families, Naididae and Tubificidae form 80 to 100% of the annelid communi- ties in the benthos of most streams and lakes at all trophic levels. They range in size from 0.1 cm in Naididae to 3 or 4 cm in relaxed length in Lumbricidae, the family that contains the earth- worms. -

Utilizing Soil Characteristics, Tissue Residues, Invertebrate Exposures
Utilizing soil characteristics, tissue residues, invertebrate exposures and invertebrate community analyses to evaluate a lead-contaminated site: A shooting range case study Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy In the Graduate School of The Ohio State University By Sarah R. Bowman, M.S. Graduate Program in Evolution, Ecology, and Organismal Biology The Ohio State University 2015 Dissertation Committee: Roman Lanno, Advisor Nicholas Basta Susan Fisher Copyright by Sarah R. Bowman 2015 Abstract With over 4,000 military shooting ranges, and approximately 9,000 non-military shooting ranges within the United States, the Department of Defense and private shooting range owners are challenged with management of these sites. Ammunition used at shooting ranges is comprised mostly of lead (Pb). Shooting ranges result in high soil metal concentrations in small areas and present unique challenges for ecological risk assessment and management. Mean natural background soil Pb is about 32 mg/kg in the eastern United States, but organisms that live in soil or in close association with soil may be at risk from elevated levels of Pb at shooting ranges. Previous shooting range studies on the ecotoxicological impacts of Pb, with few exceptions, used total soil Pb levels as a measure of exposure. However, total soil Pb levels are often not well correlated with Pb toxicity or bioaccumulation. This is a result of differences in Pb bioavailability, or the amount of Pb taken up by an organism that causes a biological response, depending on soil physical/chemical characteristics and species-specific uptake, metabolism, and elimination mechanisms. -
Size Variation and Geographical Distribution of the Luminous Earthworm Pontodrilus Litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan
A peer-reviewed open-access journal ZooKeys 862: 23–43 (2019) Size variation and distribution of Pontodrilus litoralis 23 doi: 10.3897/zookeys.862.35727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan Teerapong Seesamut1,2,4, Parin Jirapatrasilp2, Ratmanee Chanabun3, Yuichi Oba4, Somsak Panha2 1 Biological Sciences Program, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 2 Ani- mal Systematics Research Unit, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Program in Animal Science, Faculty of Agriculture Technology, Sakon Nakhon Rajabhat University, Sakon Nakhon 47000, Thailand 4 Department of Environmental Biology, Chubu University, Kasugai 487-8501, Japan Corresponding authors: Somsak Panha ([email protected]), Yuichi Oba ([email protected]) Academic editor: Samuel James | Received 24 April 2019 | Accepted 13 June 2019 | Published 9 July 2019 http://zoobank.org/663444CA-70E2-4533-895A-BF0698461CDF Citation: Seesamut T, Jirapatrasilp P, Chanabun R, Oba Y, Panha S (2019) Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. ZooKeys 862: 23–42. https://doi.org/10.3897/zookeys.862.35727 Abstract The luminous earthworm Pontodrilus litoralis (Grube, 1855) occurs in a very wide range of subtropical and tropical coastal areas. Morphometrics on size variation (number of segments, body length and diameter) and genetic analysis using the mitochondrial cytochrome c oxidase subunit 1 (COI) gene sequence were conducted on 14 populations of P. -
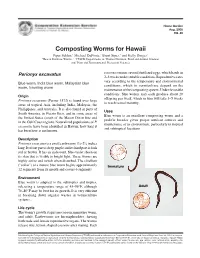
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

Phylogenetic and Phenetic Systematics of The
195 PHYLOGENETICAND PHENETICSYSTEMATICS OF THE OPISTHOP0ROUSOLIGOCHAETA (ANNELIDA: CLITELLATA) B.G.M. Janieson Departnent of Zoology University of Queensland Brisbane, Australia 4067 Received September20, L977 ABSTMCT: The nethods of Hennig for deducing phylogeny have been adapted for computer and a phylogran has been constructed together with a stereo- phylogran utilizing principle coordinates, for alL farnilies of opisthopor- ous oligochaetes, that is, the Oligochaeta with the exception of the Lunbriculida and Tubificina. A phenogran based on the sane attributes conpares unfavourably with the phyLogralnsin establishing an acceptable classification., Hennigrs principle that sister-groups be given equal rank has not been followed for every group to avoid elevation of the more plesionorph, basal cLades to inacceptabl.y high ranks, the 0ligochaeta being retained as a Subclass of the class Clitellata. Three orders are recognized: the LumbricuLida and Tubificida, which were not conputed and the affinities of which require further investigation, and the Haplotaxida, computed. The Order Haplotaxida corresponds preciseLy with the Suborder Opisthopora of Michaelsen or the Sectio Diplotesticulata of Yanaguchi. Four suborders of the Haplotaxida are recognized, the Haplotaxina, Alluroidina, Monil.igastrina and Lunbricina. The Haplotaxina and Monili- gastrina retain each a single superfanily and fanily. The Alluroidina contains the superfamiJ.y All"uroidoidea with the fanilies Alluroididae and Syngenodrilidae. The Lurnbricina consists of five superfaniLies. -

Earthworms (Annelida: Oligochaeta) of the Columbia River Basin Assessment Area
United States Department of Agriculture Earthworms (Annelida: Forest Service Pacific Northwest Oligochaeta) of the Research Station United States Columbia River Basin Department of the Interior Bureau of Land Assessment Area Management General Technical Sam James Report PNW-GTR-491 June 2000 Author Sam Jamesis an Associate Professor, Department of Life Sciences, Maharishi University of Management, Fairfield, IA 52557-1056. Earthworms (Annelida: Oligochaeta) of the Columbia River Basin Assessment Area Sam James Interior Columbia Basin Ecosystem Management Project: Scientific Assessment Thomas M. Quigley, Editor U.S. Department of Agriculture Forest Service Pacific Northwest Research Station Portland, Oregon General Technical Report PNW-GTR-491 June 2000 Preface The Interior Columbia Basin Ecosystem Management Project was initiated by the USDA Forest Service and the USDI Bureau of Land Management to respond to several critical issues including, but not limited to, forest and rangeland health, anadromous fish concerns, terrestrial species viability concerns, and the recent decline in traditional commodity flows. The charter given to the project was to develop a scientifically sound, ecosystem-based strategy for managing the lands of the interior Columbia River basin administered by the USDA Forest Service and the USDI Bureau of Land Management. The Science Integration Team was organized to develop a framework for ecosystem management, an assessment of the socioeconomic biophysical systems in the basin, and an evalua- tion of alternative management strategies. This paper is one in a series of papers developed as back- ground material for the framework, assessment, or evaluation of alternatives. It provides more detail than was possible to disclose directly in the primary documents. -

The Giant Palouse Earthworm (Driloleirus Americanus)
PETITION TO LIST The Giant Palouse Earthworm (Driloleirus americanus) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT June 30, 2009 Friends of the Clearwater Center for Biological Diversity Palouse Audubon Palouse Prairie Foundation Palouse Group of the Sierra Club 1 June 30, 2009 Ken Salazar, Secretary of the Interior Robyn Thorson, Regional Director U.S. Department of the Interior U.S. Fish & Wildlife Service 1849 C Street N.W. Pacific Region Washington, DC 20240 911 NE 11th Ave Portland, Oregon Dear Secretary Salazar, Friends of the Clearwater, Center for Biological Diversity, Palouse Prairie Foundation, Palouse Audubon, Palouse Group of the Sierra Club and Steve Paulson formally petition to list the Giant Palouse Earthworm (Driloleirus americanus) as a threatened or endangered species pursuant to the Endangered Species Act (”ESA”), 16 U.S.C. §1531 et seq. This petition is filed under 5 U.S.C. 553(e) and 50 CFR 424.14 (1990), which grant interested parties the right to petition for issuance of a rule from the Secretary of Interior. Petitioners also request that critical habitat be designated for the Giant Palouse Earthworm concurrent with the listing, pursuant to 50 CFR 424.12, and pursuant to the Administrative Procedures Act (5 U.S.C. 553). The Giant Palouse Earthworm (D. americanus) is found only in the Columbia River Drainages of eastern Washington and Northern Idaho. Only four positive collections of this species have been made within the last 110 years, despite the fact that the earthworm was historically considered “very abundant” (Smith 1897). The four collections include one between Moscow, Idaho and Pullman, Washington, one near Moscow Mountain, Idaho, one at a prairie remnant called Smoot Hill and a fourth specimen near Ellensberg, Washington (Fender and McKey- Fender, 1990, James 2000, Sánchez de León and Johnson-Maynard, 2008). -

Herpetofauna and Aquatic Macro-Invertebrate Use of the Kino Environmental Restoration Project (KERP)
Herpetofauna and Aquatic Macro-invertebrate Use of the Kino Environmental Restoration Project (KERP) Tucson, Pima County, Arizona Prepared for Pima County Regional Flood Control District Prepared by EPG, Inc. JANUARY 2007 - Plma County Regional FLOOD CONTROL DISTRICT MEMORANDUM Water Resources Regional Flood Control District DATE: January 5,2007 TO: Distribution FROM: Julia Fonseca SUBJECT: Kino Ecosystem Restoration Project Report The Ed Pastor Environmental Restoration ProjectiKino Ecosystem Restoration Project (KERP) is becoming an extraordinary urban wildlife resource. As such, the Pima County Regional Flood Control District (PCRFCD) contracted with the Environmental Planning Group (EPG) to gather observations of reptiles, amphibians, and aquatic insects at KERP. Water quality was also examined. The purpose of the work was to provide baseline data on current wildlife use of the KERP site, and to assess water quality for post-project aquatic wildlife conditions. I additionally requested sampling of macroinvertebrates at Agua Caliente Park and Sweetwater Wetlands in hopes that the differences in aquatic wildlife among the three sites might provide insights into the different habitats offered by KERF'. The results One of the most important wildlife benefits that KERP provides is aquatic habitat without predatory bullfrogs and non- native fish. Most other constructed ponds and wetlands in Tucson, such as the Sweetwater Wetlands and Agua Caliente pond, are fuIl of non-native predators which devastate native fish, amphibians and aquatic reptiles. The KERP Wetlands may provide an opportunity for reestablishing declining native herpetofauna. Provided that non- native fish, bullfrogs or crayfish are not introduced, KERP appears to provide adequate habitat for Sonoran Mud Turtles (Kinosternon sonoriense), Lowland Leopard Frogs (Rana yavapaiensis), and Mexican Gartersnakes (Tharnnophis eques) and Southwestern Woodhouse Toad (Bufo woodhousii australis). -

Checklist of New Zealand Earthworms Updated from Lee (1959) by R.J
Checklist of New Zealand Earthworms updated from Lee (1959) by R.J. Blakemore August, 2006 COE fellow, Soil Ecology Group, Yokohama National Univeristy, Japan. Summary This review is based on Blakemore (2004) that updated the work completed over 40 years earlier by Lee (1959) as modified by Blakemore in Lee et al. , (2000) and in Glasby et al. (2007/8) based on the information presented at the "Species 2000" meeting held Jan. 2000 at Te Papa Museum in Wellington, New Zealand. In the current checklist Acanthodrilidae, Octochaetidae and Megascolecidae sensu Blakemore (2000b) are all given separate family status. Whereas Lee (1959) listed approximately 193 species, the current list has about 199 taxa. Because many of the natives have few reports, or are based on only a few specimens, approximately 77 are listed as "threatened" or "endangered" in Dept. Conservation threatened species list (see www.doc.govt.nz/Conservation/001~Plants-and-Animals/006~Threatened-species/Terrestrial-invertebrate-(part-one).asp April, 2005) and three species are detailed in McGuinness (2001). Further studies such as those of Springett & Grey (1998) are required. Currently I seek funding to complete my database into an interactive guide to species, to use to conduct surveys in New Zealand. Some of the changes in Blakemore (2004) from Lee (1959) are: • Microscolex macquariensis (Beddard, 1896) is removed from the list because it is known only from Macquarie Island, which is now claimed as Australian territory (see Blakemore, 2000b). • Megascolides orthostichon (Schmarda, 1861) is removed from the fauna as Fletcher (1886: 524) reported that “on the authority of Captain Hutton” this species was not from New Zealand and may be from Mt Wellington in Tasmania (see Blakemore, 2000b). -

Arhynchobdellida (Annelida: Oligochaeta: Hirudinida): Phylogenetic Relationships and Evolution
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 30 (2004) 213–225 www.elsevier.com/locate/ympev Arhynchobdellida (Annelida: Oligochaeta: Hirudinida): phylogenetic relationships and evolution Elizabeth Bordaa,b,* and Mark E. Siddallb a Department of Biology, Graduate School and University Center, City University of New York, New York, NY, USA b Division of Invertebrate Zoology, American Museum of Natural History, New York, NY, USA Received 15 July 2003; revised 29 August 2003 Abstract A remarkable diversity of life history strategies, geographic distributions, and morphological characters provide a rich substrate for investigating the evolutionary relationships of arhynchobdellid leeches. The phylogenetic relationships, using parsimony anal- ysis, of the order Arhynchobdellida were investigated using nuclear 18S and 28S rDNA, mitochondrial 12S rDNA, and cytochrome c oxidase subunit I sequence data, as well as 24 morphological characters. Thirty-nine arhynchobdellid species were selected to represent the seven currently recognized families. Sixteen rhynchobdellid leeches from the families Glossiphoniidae and Piscicolidae were included as outgroup taxa. Analysis of all available data resolved a single most-parsimonious tree. The cladogram conflicted with most of the traditional classification schemes of the Arhynchobdellida. Monophyly of the Erpobdelliformes and Hirudini- formes was supported, whereas the families Haemadipsidae, Haemopidae, and Hirudinidae, as well as the genera Hirudo or Ali- olimnatis, were found not to be monophyletic. The results provide insight on the phylogenetic positions for the taxonomically problematic families Americobdellidae and Cylicobdellidae, the genera Semiscolex, Patagoniobdella, and Mesobdella, as well as genera traditionally classified under Hirudinidae. The evolution of dietary and habitat preferences is examined. Ó 2003 Elsevier Inc. All rights reserved.