Phylogeny of the Megascolecidae and Crassiclitellata (Annelida
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomic Assessment of Lumbricidae (Oligochaeta) Earthworm Genera Using DNA Barcodes
European Journal of Soil Biology 48 (2012) 41e47 Contents lists available at SciVerse ScienceDirect European Journal of Soil Biology journal homepage: http://www.elsevier.com/locate/ejsobi Original article Taxonomic assessment of Lumbricidae (Oligochaeta) earthworm genera using DNA barcodes Marcos Pérez-Losada a,*, Rebecca Bloch b, Jesse W. Breinholt c, Markus Pfenninger b, Jorge Domínguez d a CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, 4485-661 Vairão, Portugal b Biodiversity and Climate Research Centre, Lab Centre, Biocampus Siesmayerstraße, 60323 Frankfurt am Main, Germany c Department of Biology, Brigham Young University, Provo, UT 84602-5181, USA d Departamento de Ecoloxía e Bioloxía Animal, Universidade de Vigo, E-36310, Spain article info abstract Article history: The family Lumbricidae accounts for the most abundant earthworms in grasslands and agricultural Received 26 May 2011 ecosystems in the Paleartic region. Therefore, they are commonly used as model organisms in studies of Received in revised form soil ecology, biodiversity, biogeography, evolution, conservation, soil contamination and ecotoxicology. 14 October 2011 Despite their biological and economic importance, the taxonomic status and evolutionary relationships Accepted 14 October 2011 of several Lumbricidae genera are still under discussion. Previous studies have shown that cytochrome c Available online 30 October 2011 Handling editor: Stefan Schrader oxidase I (COI) barcode phylogenies are informative at the intrageneric level. Here we generated 19 new COI barcodes for selected Aporrectodea specimens in Pérez-Losada et al. [1] including nine species and 17 Keywords: populations, and combined them with all the COI sequences available in Genbank and Briones et al. -

Earthworm, Suborder Crassiclitellata, Cohort Terrimegadrili (Jamieson, 1988)1 William T
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-532 Earthworm, suborder Crassiclitellata, cohort Terrimegadrili (Jamieson, 1988)1 William T. Crow2 Introduction The term earthworm is commonly assigned to certain worms in the class Clitellata in the phylum Annelida. Like insects, earthworms (Fig. 1) are among the animals Annelid worms are distinguished from other important most frequently encountered by many Floridians. Our kids worms like nematodes by having a coelum or true body play with them (Fig. 2 A,B), dissect them in middle school cavity, a circulatory system, and a body divided into biology, we fish with them, they crawl across our sidewalks segments. Other familiar annelids are the Hirudinea and live in our flower pots. Despite this, their ecological (leeches), the Polychaeta (marine bristleworms), and the and economic importance often goes unrecognized. Enchytraeids (potworms). Within the order Opisthopora Earthworms have several important ecological roles. there are both aquatic and terrestrial species. We will use Additionally, some species are used commercially for bait, earthworm exclusively for terrestrial worms in the subor- animal feed, environmental remediation, and composting. der Crassiclitellata. Figure 2. Earthworms are frequently encountered by many Floridians. Credits: A) Emily E. Eubanks, University of Florida. B) William T. Crow, Figure 1. Earthworms collected from a parking lot following a heavy University of Florida rainfall. Credits: William T. Crow, University of Florida 1. This document is EENY-532, one of a series of the Entomology and Nematology Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

Physical, Nutritional and Biochemical Status of Vermiwash Produced by Two Earthworm Species Lampito Mauritii (L) and Eudrillus Eugeniae (L)
Available online at www.worldscientificnews.com WSN 42 (2016) 228-255 EISSN 2392-2192 Physical, nutritional and biochemical status of vermiwash produced by two earthworm species Lampito mauritii (L) and Eudrillus eugeniae (L). Vitthalrao B. Khyade1,*, Sunanda Rajendra Pawar2 1The Research Group, Agriculture Development Trust, Shardanagar, Malegaon (Baramati) Dist. Pune – 413115, India 2Trustee and Head Academic Section, Agricultural Development Trust, Baramati Shardanagar, (Malegaon Col.) Post Box No.- 35, Tal. Baramati. Dist. Pune - 413 115 Maharashtra, India *E-mail address: [email protected] ABSTRACT In vermiculture, it is mandatory to keep the feed given to earthworm moist which will enable them to eat and procreate. Water is regularly sprinkled over the feed. The water mixes in the feed and oily content of earthworms body and slowly drains out from earthworm beds. The outgoing liquid is a concentrate with nutrients which is very beneficial for plants growth. This liquid is called vermiwash. The vermiwash is potential application in sustainable development for agriculture and biotechnology. This attempt deals with assessment the physico-chemical, nutritional and biochemical status of the vermiwash obtained using the popular composting earthworm species Eudrillus eugeniae (Kinb.) (Eudrilidae: Haplotaxida) and Lampito mauritii from three different leaf litters namely, Mango (Mangifera indica), Guava (Psidium guajava) and Sapota (Achrus sapota). The results showed substantial increase in the nutrient quality of the vermiwash produced with time in all of three cases. However, the vermiwash produced from guava leaf litter showed more content of electrical conductivity, magnesium, calcium, nitrite, phosphorus, carbohydrate, protein, lipid and amino acid compared with the vermiwash produced from the other two sapota and mango leaf litter by using the both earthworm species Eudrillus eugeniae and Lampito mauritii respectively. -

French Mediterranean Islands As a Refuge of Relic Earthworm Species: Cataladrilus Porquerollensis Sp
French Mediterranean islands as a refuge of relic earthworm species: Cataladrilus porquerollensis sp. nov. and Scherotheca portcrosana sp. nov. (Crassiclitellata, Lumbricidae) Daniel F Marchan, Thibaud Decaens, Darío J. Diaz Cosin, Mickael Hedde, Emmanuel Lapied, Jorge Domínguez To cite this version: Daniel F Marchan, Thibaud Decaens, Darío J. Diaz Cosin, Mickael Hedde, Emmanuel Lapied, et al.. French Mediterranean islands as a refuge of relic earthworm species: Cataladrilus porquerollensis sp. nov. and Scherotheca portcrosana sp. nov. (Crassiclitellata, Lumbricidae). European Journal of Taxonomy, Consortium of European Natural History Museums, 2020, 10.5852/ejt.2020.701. hal- 02940303 HAL Id: hal-02940303 https://hal.inrae.fr/hal-02940303 Submitted on 16 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. European Journal of Taxonomy 701: 1–22 ISSN 2118-9773 https://doi.org/10.5852/ejt.2020.701 www.europeanjournaloftaxonomy.eu 2020 · Marchán D.F. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:D9291955-F619-46EA-90E1-DA756D1B7C55 French Mediterranean islands as a refuge of relic earthworm species: Cataladrilus porquerollensis sp. nov. and Scherotheca portcrosana sp. -

Species Diversity of Terrestrial Earthworms in Different
SPECIES DIVERSITY OF TERRESTRIAL EARTHWORMS IN KHAO YAI NATIONAL PARK Prasuk Kosavititkul A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Environmental Biology Suranaree University of Technology Academic Year 2005 ISBN 974-533-516-9 ความหลากหลายของชนิดไสเดือนดินในเขตอุทยานแหงชาติเขาใหญ นายประสุข โฆษวิฑิตกุล วิทยานิพนธน เปี้ นสวนหนงของการศึ่ ึกษาตามหลักสูตรปริญญาวิทยาศาสตรดุษฎีบณฑั ิต สาขาวิชาชีววทยาสิ ิ่งแวดลอม มหาวิทยาลัยเทคโนโลยีสุรนารี ปการศึกษา 2548 ISBN 974-533-516-9 ACKNOWLEDGEMENTS I am most grateful to thank Asst. Prof. Dr. Panee Wannitikul, my advisor, for her generous help, encouragement and guidance throughout this thesis from the beginning. Her criticism, improvement, and proper of manuscript have made this thesis in correct form. I sincerely thank to Assoc. Prof. Dr. Korakod Indrapichate, Assoc. Prof. Dr. Somsak Panha, Dr. Nathawut Thanee and Dr. Pongthep Suwanwaree for their valuable advice and guidance in this thesis. I am sincerely grateful to Assoc. Prof. Dr. Sam James, Natural History Museum and Biodiversity Research Center, Kansas University for his helpful and kind assistance in confirming and identifying earthworms. Thanks also to the Center for Scientific and Technological Equipment, Suranaree University of Technology for the laboratory facilities and scientific instruments. Special thank is due to the Khao Yai National Park for permitting me to work in the park. I would like to express my special thank to Naresuan University for the scholarship supporting my study. Special gratitude is expressed to my parents, my sisters, my family, my friends, my seniors, my juniors, and other people who give me a supported power whenever I lose my own power. Prasuk Kosavititkul CONTENTS Page ABSTRACT IN THAI…………………………………………………………… I ABSTRACT IN ENGLISH……………………………………………………… II ACKNOWLEDGEMENTS…………………………………………………….. -

Morphological and Histological Studies on the Vermicomposting Indian Earthworm Eudrilus Eugeniae
World Journal of Zoology 7 (2): 165-170, 2012 ISSN 1817-3098 © IDOSI Publications, 2012 DOI: 10.5829/idosi.wjz.2012.7.2.62154 Morphological and Histological Studies on the Vermicomposting Indian Earthworm Eudrilus eugeniae 12T.M. Vijaya, Sushil Kumar Middha, 3Talambedu Usha, 1H.K. Aruna, 12R. Bharathi, Deepti Saini and 4G. Govindaraj 1Department of Zoology, Maharani Lakshmi Ammanni College For Women, Malleswaram, Bangalore - 560012, India 2Department of Biotechnology, Maharani Lakshmi Ammanni College For Women, Malleswaram, Bangalore - 560012, India 3Department of Biochemistry, Maharani Lakshmi Ammanni College For Women, Malleswaram, Bangalore - 560012, India 4Department of Entomology, University of Agricultural Sciences, G.K.V.K. Bangalore - 560065, India Abstract: Earthworm is a potential contributor in organic waste disposal or vermicomposting. Eudrilus eugeniae collected from moist subsurface soil and under stones in the University of Agriculture Sciences, GKVK, Bangalore, India, were studied. E. eugeniae can also be utilized for protein source in animal feed. The external features, growth, reproductive morphology and histology were investigated. The Indian E. eugeniae has higher bodyweight as compared to the African counterparts, in spite of comparable lengths. Morphology of the reproductive parts and histology of the ovary and oviduct of these worms are elucidated by histological staining methods. There have been no previous reports about these clitellar earthworms from the Indian subcontinent. The histological details of the ovary reveals the presence of larger follicles towards the periphery which shows degenerative changes, while the smaller primary follicles and oocyte are concentrated in the center. Further, the posterior part of the oviduct shows the presence of dense mass of sperms in its large lumen which confirms the process of internal fertilization in Eudrilus eugeniae. -

Redalyc.CONTINENTAL BIODIVERSITY of SOUTH
Acta Zoológica Mexicana (nueva serie) ISSN: 0065-1737 [email protected] Instituto de Ecología, A.C. México Christoffersen, Martin Lindsey CONTINENTAL BIODIVERSITY OF SOUTH AMERICAN OLIGOCHAETES: THE IMPORTANCE OF INVENTORIES Acta Zoológica Mexicana (nueva serie), núm. 2, 2010, pp. 35-46 Instituto de Ecología, A.C. Xalapa, México Available in: http://www.redalyc.org/articulo.oa?id=57515556003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ISSN 0065-1737 Acta ZoológicaActa Zoológica Mexicana Mexicana (n.s.) Número (n.s.) Número Especial Especial 2: 35-46 2 (2010) CONTINENTAL BIODIVERSITY OF SOUTH AMERICAN OLIGOCHAETES: THE IMPORTANCE OF INVENTORIES Martin Lindsey CHRISTOFFERSEN Universidade Federal da Paraíba, Departamento de Sistemática e Ecologia, 58.059-900, João Pessoa, Paraíba, Brasil. E-mail: [email protected] Christoffersen, M. L. 2010. Continental biodiversity of South American oligochaetes: The importance of inventories. Acta Zoológica Mexicana (n.s.), Número Especial 2: 35-46. ABSTRACT. A reevaluation of South American oligochaetes produced 871 known species. Megadrile earthworms have rates of endemism around 90% in South America, while Enchytraeidae have less than 75% endemism, and aquatic oligochaetes have less than 40% endemic taxa in South America. Glossoscolecid species number 429 species in South America alone, a full two-thirds of the known megadrile earthworms. More than half of the South American taxa of Oligochaeta (424) occur in Brazil, being followed by Argentina (208 taxa), Ecuador (163 taxa), and Colombia (142 taxa). -
Size Variation and Geographical Distribution of the Luminous Earthworm Pontodrilus Litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan
A peer-reviewed open-access journal ZooKeys 862: 23–43 (2019) Size variation and distribution of Pontodrilus litoralis 23 doi: 10.3897/zookeys.862.35727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan Teerapong Seesamut1,2,4, Parin Jirapatrasilp2, Ratmanee Chanabun3, Yuichi Oba4, Somsak Panha2 1 Biological Sciences Program, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 2 Ani- mal Systematics Research Unit, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Program in Animal Science, Faculty of Agriculture Technology, Sakon Nakhon Rajabhat University, Sakon Nakhon 47000, Thailand 4 Department of Environmental Biology, Chubu University, Kasugai 487-8501, Japan Corresponding authors: Somsak Panha ([email protected]), Yuichi Oba ([email protected]) Academic editor: Samuel James | Received 24 April 2019 | Accepted 13 June 2019 | Published 9 July 2019 http://zoobank.org/663444CA-70E2-4533-895A-BF0698461CDF Citation: Seesamut T, Jirapatrasilp P, Chanabun R, Oba Y, Panha S (2019) Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. ZooKeys 862: 23–42. https://doi.org/10.3897/zookeys.862.35727 Abstract The luminous earthworm Pontodrilus litoralis (Grube, 1855) occurs in a very wide range of subtropical and tropical coastal areas. Morphometrics on size variation (number of segments, body length and diameter) and genetic analysis using the mitochondrial cytochrome c oxidase subunit 1 (COI) gene sequence were conducted on 14 populations of P. -

New Light Into the Hormogastrid Riddle: Morphological and Molecular Description of Hormogaster Joseantonioi Sp. N. (Annelida
New light into the hormogastrid riddle: morphological and molecular description of Hormogaster joseantonioi sp. n. (Annelida, Clitellata, Hormogastridae) The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Fernández Marchán, Daniel, Rosa Fernández, Marta Novo, and Darío J. Díaz Cosín. 2014. “New light into the hormogastrid riddle: morphological and molecular description of Hormogaster joseantonioi sp. n. (Annelida, Clitellata, Hormogastridae).” ZooKeys (414): 1-17. doi:10.3897/zookeys.414.7665. http://dx.doi.org/10.3897/zookeys.414.7665. Published Version doi:10.3897/zookeys.414.7665 Accessed February 16, 2015 3:42:37 PM EST Citable Link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12717446 Terms of Use This article was downloaded from Harvard University's DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http://nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA (Article begins on next page) A peer-reviewed open-access journal ZooKeys 414:New 1–17 light(2014) into the hormogastrid riddle: morphological and molecular description... 1 doi: 10.3897/zookeys.414.7665 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research New light into the hormogastrid riddle: morphological and molecular description of Hormogaster joseantonioi sp. n. (Annelida, Clitellata, Hormogastridae) Daniel Fernández Marchán1,†, Rosa Fernández2,‡, Marta Novo3,§, Darío J. Díaz Cosín1,| -

French Mediterranean Islands As a Refuge of Relic Earthworm Species: Cataladrilus Porquerollensis Sp
European Journal of Taxonomy 701: 1–22 ISSN 2118-9773 https://doi.org/10.5852/ejt.2020.701 www.europeanjournaloftaxonomy.eu 2020 · Marchán D.F. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:D9291955-F619-46EA-90E1-DA756D1B7C55 French Mediterranean islands as a refuge of relic earthworm species: Cataladrilus porquerollensis sp. nov. and Scherotheca portcrosana sp. nov. (Crassiclitellata, Lumbricidae) Daniel F. MARCHÁN 1,3,*, Thibaud DECAËNS 2,*, Darío J. DÍAZ COSÍN 3, Mickaël HEDDE 4, Emmanuel LAPIED 5 & Jorge DOMÍNGUEZ 6 1,6 Grupo de Ecoloxía Animal (GEA), Universidade de Vigo, E-36310 Vigo, Spain. 2 CEFE, Univ Montpellier, CNRS, EPHE, IRD, Univ Paul Valéry Montpellier 3, Montpellier, France. 3 Department of Biodiversity, Ecology and Evolution, Faculty of Biology, Universidad Complutense de Madrid, Madrid, Spain. 4 UMR Eco&Sols, INRAE–IRD–CIRAD–SupAgro Montpellier, 2 Place Viala, 34060 Montpellier Cedex 2, France. 5 Taxonomia Biodiversity Fund, 7 rue Beccaria, 72012, Paris, France. * Corresponding authors: [email protected]; [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 6 Email: [email protected] 1 urn:lsid:zoobank.org:author:3B3731B6-B5FB-409A-A7A3-99FD0F96D688 2 urn:lsid:zoobank.org:author:B61F61B2-3012-4526-8FF9-DC94D372AF77 3 urn:lsid:zoobank.org:author:38538B17-F127-4438-9DE2-F9D6C597D044 4 urn:lsid:zoobank.org:author:F4A219F7-7E75-4333-8293-3004B3CD62C5 5 urn:lsid:zoobank.org:author:B1FB8658-DFC3-481C-A0BE-B8488A018611 6 urn:lsid:zoobank.org:author:167575D5-D2CC-4B37-8B1D-0233E6B154E5 Abstract. -
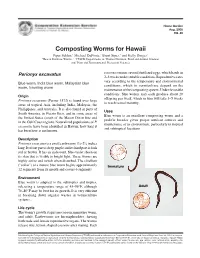
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

Phylogenetic and Phenetic Systematics of The
195 PHYLOGENETICAND PHENETICSYSTEMATICS OF THE OPISTHOP0ROUSOLIGOCHAETA (ANNELIDA: CLITELLATA) B.G.M. Janieson Departnent of Zoology University of Queensland Brisbane, Australia 4067 Received September20, L977 ABSTMCT: The nethods of Hennig for deducing phylogeny have been adapted for computer and a phylogran has been constructed together with a stereo- phylogran utilizing principle coordinates, for alL farnilies of opisthopor- ous oligochaetes, that is, the Oligochaeta with the exception of the Lunbriculida and Tubificina. A phenogran based on the sane attributes conpares unfavourably with the phyLogralnsin establishing an acceptable classification., Hennigrs principle that sister-groups be given equal rank has not been followed for every group to avoid elevation of the more plesionorph, basal cLades to inacceptabl.y high ranks, the 0ligochaeta being retained as a Subclass of the class Clitellata. Three orders are recognized: the LumbricuLida and Tubificida, which were not conputed and the affinities of which require further investigation, and the Haplotaxida, computed. The Order Haplotaxida corresponds preciseLy with the Suborder Opisthopora of Michaelsen or the Sectio Diplotesticulata of Yanaguchi. Four suborders of the Haplotaxida are recognized, the Haplotaxina, Alluroidina, Monil.igastrina and Lunbricina. The Haplotaxina and Monili- gastrina retain each a single superfanily and fanily. The Alluroidina contains the superfamiJ.y All"uroidoidea with the fanilies Alluroididae and Syngenodrilidae. The Lurnbricina consists of five superfaniLies.