MEGADRILOGICA Volume 16, Number 2, February 2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, and HABITAT DEGRADATION REDUCE SALAMANDER DENSITY Julie L
John Carroll University Carroll Collected Masters Theses Theses, Essays, and Senior Honors Projects Summer 2015 INVASIVE ASIAN EARTHWORMS NEGATIVELY IMPACT WOODLAND SALAMANDERS: COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, AND HABITAT DEGRADATION REDUCE SALAMANDER DENSITY Julie L. Ziemba John Carroll University, [email protected] Follow this and additional works at: http://collected.jcu.edu/masterstheses Part of the Biology Commons Recommended Citation Ziemba, Julie L., "INVASIVE ASIAN EARTHWORMS NEGATIVELY IMPACT WOODLAND SALAMANDERS: COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, AND HABITAT DEGRADATION REDUCE SALAMANDER DENSITY" (2015). Masters Theses. 13. http://collected.jcu.edu/masterstheses/13 This Thesis is brought to you for free and open access by the Theses, Essays, and Senior Honors Projects at Carroll Collected. It has been accepted for inclusion in Masters Theses by an authorized administrator of Carroll Collected. For more information, please contact [email protected]. INVASIVE ASIAN EARTHWORMS NEGATIVELY IMPACT WOODLAND SALAMANDERS: COMPETITIVE EXCLUSION, FORAGING INTERFERENCE, AND HABITAT DEGRADATION REDUCE SALAMANDER DENSITY A Thesis Submitted to the Office of Graduate Studies College of Arts & Sciences of John Carroll University in Partial Fulfillment of the Requirements for the Degree of Master of Science By Julie L. Ziemba 2015 Table of Contents Abstract ..........................................................................................................................1 1. Introduction ................................................................................................................3 -
Size Variation and Geographical Distribution of the Luminous Earthworm Pontodrilus Litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan
A peer-reviewed open-access journal ZooKeys 862: 23–43 (2019) Size variation and distribution of Pontodrilus litoralis 23 doi: 10.3897/zookeys.862.35727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan Teerapong Seesamut1,2,4, Parin Jirapatrasilp2, Ratmanee Chanabun3, Yuichi Oba4, Somsak Panha2 1 Biological Sciences Program, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 2 Ani- mal Systematics Research Unit, Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand 3 Program in Animal Science, Faculty of Agriculture Technology, Sakon Nakhon Rajabhat University, Sakon Nakhon 47000, Thailand 4 Department of Environmental Biology, Chubu University, Kasugai 487-8501, Japan Corresponding authors: Somsak Panha ([email protected]), Yuichi Oba ([email protected]) Academic editor: Samuel James | Received 24 April 2019 | Accepted 13 June 2019 | Published 9 July 2019 http://zoobank.org/663444CA-70E2-4533-895A-BF0698461CDF Citation: Seesamut T, Jirapatrasilp P, Chanabun R, Oba Y, Panha S (2019) Size variation and geographical distribution of the luminous earthworm Pontodrilus litoralis (Grube, 1855) (Clitellata, Megascolecidae) in Southeast Asia and Japan. ZooKeys 862: 23–42. https://doi.org/10.3897/zookeys.862.35727 Abstract The luminous earthworm Pontodrilus litoralis (Grube, 1855) occurs in a very wide range of subtropical and tropical coastal areas. Morphometrics on size variation (number of segments, body length and diameter) and genetic analysis using the mitochondrial cytochrome c oxidase subunit 1 (COI) gene sequence were conducted on 14 populations of P. -
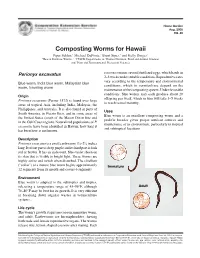
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

Phylogenetic and Phenetic Systematics of The
195 PHYLOGENETICAND PHENETICSYSTEMATICS OF THE OPISTHOP0ROUSOLIGOCHAETA (ANNELIDA: CLITELLATA) B.G.M. Janieson Departnent of Zoology University of Queensland Brisbane, Australia 4067 Received September20, L977 ABSTMCT: The nethods of Hennig for deducing phylogeny have been adapted for computer and a phylogran has been constructed together with a stereo- phylogran utilizing principle coordinates, for alL farnilies of opisthopor- ous oligochaetes, that is, the Oligochaeta with the exception of the Lunbriculida and Tubificina. A phenogran based on the sane attributes conpares unfavourably with the phyLogralnsin establishing an acceptable classification., Hennigrs principle that sister-groups be given equal rank has not been followed for every group to avoid elevation of the more plesionorph, basal cLades to inacceptabl.y high ranks, the 0ligochaeta being retained as a Subclass of the class Clitellata. Three orders are recognized: the LumbricuLida and Tubificida, which were not conputed and the affinities of which require further investigation, and the Haplotaxida, computed. The Order Haplotaxida corresponds preciseLy with the Suborder Opisthopora of Michaelsen or the Sectio Diplotesticulata of Yanaguchi. Four suborders of the Haplotaxida are recognized, the Haplotaxina, Alluroidina, Monil.igastrina and Lunbricina. The Haplotaxina and Monili- gastrina retain each a single superfanily and fanily. The Alluroidina contains the superfamiJ.y All"uroidoidea with the fanilies Alluroididae and Syngenodrilidae. The Lurnbricina consists of five superfaniLies. -

The Giant Palouse Earthworm (Driloleirus Americanus)
PETITION TO LIST The Giant Palouse Earthworm (Driloleirus americanus) AS A THREATENED OR ENDANGERED SPECIES UNDER THE ENDANGERED SPECIES ACT June 30, 2009 Friends of the Clearwater Center for Biological Diversity Palouse Audubon Palouse Prairie Foundation Palouse Group of the Sierra Club 1 June 30, 2009 Ken Salazar, Secretary of the Interior Robyn Thorson, Regional Director U.S. Department of the Interior U.S. Fish & Wildlife Service 1849 C Street N.W. Pacific Region Washington, DC 20240 911 NE 11th Ave Portland, Oregon Dear Secretary Salazar, Friends of the Clearwater, Center for Biological Diversity, Palouse Prairie Foundation, Palouse Audubon, Palouse Group of the Sierra Club and Steve Paulson formally petition to list the Giant Palouse Earthworm (Driloleirus americanus) as a threatened or endangered species pursuant to the Endangered Species Act (”ESA”), 16 U.S.C. §1531 et seq. This petition is filed under 5 U.S.C. 553(e) and 50 CFR 424.14 (1990), which grant interested parties the right to petition for issuance of a rule from the Secretary of Interior. Petitioners also request that critical habitat be designated for the Giant Palouse Earthworm concurrent with the listing, pursuant to 50 CFR 424.12, and pursuant to the Administrative Procedures Act (5 U.S.C. 553). The Giant Palouse Earthworm (D. americanus) is found only in the Columbia River Drainages of eastern Washington and Northern Idaho. Only four positive collections of this species have been made within the last 110 years, despite the fact that the earthworm was historically considered “very abundant” (Smith 1897). The four collections include one between Moscow, Idaho and Pullman, Washington, one near Moscow Mountain, Idaho, one at a prairie remnant called Smoot Hill and a fourth specimen near Ellensberg, Washington (Fender and McKey- Fender, 1990, James 2000, Sánchez de León and Johnson-Maynard, 2008). -

Checklist of New Zealand Earthworms Updated from Lee (1959) by R.J
Checklist of New Zealand Earthworms updated from Lee (1959) by R.J. Blakemore August, 2006 COE fellow, Soil Ecology Group, Yokohama National Univeristy, Japan. Summary This review is based on Blakemore (2004) that updated the work completed over 40 years earlier by Lee (1959) as modified by Blakemore in Lee et al. , (2000) and in Glasby et al. (2007/8) based on the information presented at the "Species 2000" meeting held Jan. 2000 at Te Papa Museum in Wellington, New Zealand. In the current checklist Acanthodrilidae, Octochaetidae and Megascolecidae sensu Blakemore (2000b) are all given separate family status. Whereas Lee (1959) listed approximately 193 species, the current list has about 199 taxa. Because many of the natives have few reports, or are based on only a few specimens, approximately 77 are listed as "threatened" or "endangered" in Dept. Conservation threatened species list (see www.doc.govt.nz/Conservation/001~Plants-and-Animals/006~Threatened-species/Terrestrial-invertebrate-(part-one).asp April, 2005) and three species are detailed in McGuinness (2001). Further studies such as those of Springett & Grey (1998) are required. Currently I seek funding to complete my database into an interactive guide to species, to use to conduct surveys in New Zealand. Some of the changes in Blakemore (2004) from Lee (1959) are: • Microscolex macquariensis (Beddard, 1896) is removed from the list because it is known only from Macquarie Island, which is now claimed as Australian territory (see Blakemore, 2000b). • Megascolides orthostichon (Schmarda, 1861) is removed from the fauna as Fletcher (1886: 524) reported that “on the authority of Captain Hutton” this species was not from New Zealand and may be from Mt Wellington in Tasmania (see Blakemore, 2000b). -

Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems
Colby College Digital Commons @ Colby Honors Theses Student Research 2019 Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems Ella L. Maddi Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Environmental Sciences Commons, and the Soil Science Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Maddi, Ella L., "Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems" (2019). Honors Theses. Paper 965. https://digitalcommons.colby.edu/honorstheses/965 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented to the Faculty of the Department of Biology at Colby College in partial fulfillment of the requirements for the Degree of Bachelor of Arts with Honors By Ella Maddi Waterville, ME May 20, 2019 Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented -

Earthworm Ecology from DARWIN to VERMICUL TURE for 1882
Earthworm Ecology FROM DARWIN TO VERMICUL TURE FOR 1882. MAN ·I~ ·BVT ·A·woR...JV\· Frontispiece Cartoon from Punch, December 6th, 1881 Earthworm Ecology FROM DARWIN TO VERMICUL TURE Edited by J. E. Satchell Institute of Terrestrial Ecology Merlewood Research Station Grange-over-Sands Cumbria, UK LONDON NEW YORK CHAPMAN AND HALL First published 1983 by Chapman and Hall Ltd I I New Fetter Lane, London EC4P 4EE Published in the USA by Chapman and Hall 733 Third Avenue, New York NY100I7 © 1983 Chapman and Hall Ltd Softcover reprint of the hardcover 1st edition 2007 University Press, Cambridge ISBN-13: 978-94-009-5967-5 e-ISBN-13: 978-94-009-5965-1 DOl: 10.1007/978-94-009-5965-1 All rights reserved. No part ofthis book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and record ing, or in any information storage and retrieval system, without permission in writing from the Publisher. British Library Cataloguing in Publication Data Earthworm ecology. I. Opisthopora I. Satchell, J. E. 595.I' 46 QL39 I. 04 Library of Congress Cataloging in Publication Data Main entry under title: Earthworm ecology. Bibliography: p. Includes index. I. Opisthopora-Ecology. 2. Earthworm culture. I. Satchell, John E. QL39I.A6E2 7 1983 Contents Preface Xl Contributors xiii DARWIN'S CONTRIBUTION TO EARTHWORM ECOLOGY I Darwin's Formation of Vegetable Mould- its philo sophical basis M. S. Ghilarov I 2 D~rwin on earthworms - the contemporary back ground and what the critics thought O. -

Ecological Soil Screening Levels for Copper. Interim Final
Ecological Soil Screening Levels for Copper Interim Final OSWER Directive 9285.7-68 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 Issued July 2006 Revised February 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR COPPER...................................1 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................4 4.0 ECO-SSL FOR SOIL INVERTEBRATES....................................4 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................9 5.1 Avian TRV ........................................................9 5.2 Estimation of Dose and Calculation of the Eco-SSL .......................18 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE .................................18 6.1 Mammalian TRV ..................................................18 6.2 Estimation of Dose and Calculation of the Eco-SSL .......................24 7.0 REFERENCES .........................................................26 7.1 General Copper References ..........................................26 7.2 References for Plants and Soil Invertebrates .............................27 7.3 References Rejected for Use in Deriving Plant and Soil Invertebrate Eco-SSLs ...............................................................29 7.4 References Used in Deriving Wildlife TRVs ............................56 7.5 References Rejected for Use in Derivation of Wildlife TRV -

Diplopoda) of Twelve Caves in Western Mecsek, Southwest Hungary
Opusc. Zool. Budapest, 2013, 44(2): 99–106 Millipedes (Diplopoda) of twelve caves in Western Mecsek, Southwest Hungary D. ANGYAL & Z. KORSÓS Dorottya Angyal and Dr. Zoltán Korsós, Department of Zoology, Hungarian Natural History Museum, H-1088 Budapest, Baross u. 13., E-mails: [email protected], [email protected] Abstract. Twelve caves of Western Mecsek, Southwest Hungary were examined between September 2010 and April 2013 from the millipede (Diplopoda) faunistical point of view. Ten species were found in eight caves, which consisted eutroglophile and troglobiont elements as well. The cave with the most diverse fauna was the Törökpince Sinkhole, while the two previously also investigated caves, the Abaligeti Cave and the Mánfai-kőlyuk Cave provided less species, which could be related to their advanced touristic and industrial utilization. Keywords. Diplopoda, Mecsek Mts., caves, faunistics INTRODUCTION proved to be rather widespread in the karstic regions of the former Yugoslavia (Mršić 1998, lthough more than 220 caves are known 1994, Ćurčić & Makarov 1998), the species was A from the Mecsek Mts., our knowledge on the not yet found in other Hungarian caves. invertebrate fauna of the caves in the region is rather poor. Only two caves, the Abaligeti Cave All the six millipede species of the Mánfai- and the Mánfai-kőlyuk Cave have previously been kőlyuk Cave (Polyxenus lagurus (Linnaeus, examined in speleozoological studies which in- 1758), Glomeris hexasticha Brandt, 1833, Hap- cludeed the investigation of the diplopod fauna as loporatia sp., Polydesmus collaris C. L. Koch, well (Bokor 1924, Verhoeff 1928, Gebhardt 1847, Ommatoiulus sabulosus (Linnaeus, 1758) and Leptoiulus sp.) were found in the entrance 1933a, 1933b, 1934, 1963, 1966, Farkas 1957). -

A Comparative Study on the Hemoglobins and Intestinal Isozymes of Three Species of Earthworms
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Fall 1976 A COMPARATIVE STUDY ON THE HEMOGLOBINS AND INTESTINAL ISOZYMES OF THREE SPECIES OF EARTHWORMS KENNETH VASKEN KALOUSTIAN Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation KALOUSTIAN, KENNETH VASKEN, "A COMPARATIVE STUDY ON THE HEMOGLOBINS AND INTESTINAL ISOZYMES OF THREE SPECIES OF EARTHWORMS" (1976). Doctoral Dissertations. 1132. https://scholars.unh.edu/dissertation/1132 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

An Integrative Taxonomic Approach to the Identification of Three New New Zealand Endemic Earthworm Species (Acanthodrilidae, Octochaetidae: Oligochaeta)
Zootaxa 2994: 21–32 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) An integrative taxonomic approach to the identification of three new New Zealand endemic earthworm species (Acanthodrilidae, Octochaetidae: Oligochaeta) STEPHANE BOYER1,3, ROBERT J. BLAKEMORE2 & STEVE D. WRATTEN1 1Bio-Protection Research Centre, Lincoln University, New Zealand 2National Museum of Science and Nature in Tokyo, Japan 3Corresponding author. E-mail: [email protected] Abstract This work adds three new species to the ca. 200 currently known from New Zealand. In Acanthodrilidae is Maoridrilus felix and in Octochaetidae are Deinodrilus gorgon and Octochaetus kenleei. All three are endemics that often have restrict- ed ranges; however, little is yet known of their distribution, ecology nor conservation status. DNA barcoding was conduct- ed, which is the first time that New Zealand endemic holotypes have been so characterized. The barcoding region COI (cytochrome c oxidase subunit 1) as well as the 16S rDNA region were sequenced using tissue from the holotype specimen to provide indisputable uniqueness of the species. These DNA sequences are publically available on GenBank to allow accurate cross checking to verify the identification of other specimens or even to identify specimens on the basis of their DNA sequences alone. Based on their 16S rDNA sequences, the position of the three newly described species in the phy- logeny of New Zealand earthworms was discussed. The description of new species using this approach is encouraged, to provide a user-friendly identification tool for ecologists studying diverse endemic faunas of poorly known earthworm species.