Biogeography Meets Taxonomy: Distribution-Based Inferences on the Accuracy of Identification and Synonymization of East Asian Earthworms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Three New “Caecate” Earthworm Species from Sulawesi, Indonesia (Oligochaeta, Megascolecidae)
A peer-reviewed open-access journal ZooKeys 805: 1–14 (2018)Three new “caecate” earthworm species from Sulawesi, Indonesia 1 doi: 10.3897/zookeys.805.24834 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Three new “caecate” earthworm species from Sulawesi, Indonesia (Oligochaeta, Megascolecidae) Fahri Fahri1, Rizki Amaliah1, Bambang Suryobroto2, Tri Atmowidi2, Anh D. Nguyen3 1 Department of Biology, Faculty of Mathematics and Natural Sciences, Tadulako University, Jalan Raya Soekarno–Hatta, Tondo, Palu, 94117, Central Sulawesi, Indonesia 2 Department of Biology, Faculty of Ma- thematics and Natural Sciences, Bogor Agricultural University, Dramaga Campus, Bogor 16680, Indonesia 3 Duy Tan University, 254, Nguyen Van Linh, Da Nang city, Vietnam Corresponding author: Fahri Fahri ([email protected]) Academic editor: S. James | Received 6 March 2018 | Accepted 23 October 2018 | Published 11 December 2018 http://zoobank.org/888EA04C-6C61-479D-A134-96125528D032 Citation: Fahri F, Amaliah R, Suryobroto B, Atmowidi T, Nguyen AD (2018) Three new “caecate” earthworm species from Sulawesi, Indonesia (Oligochaeta, Megascolecidae). ZooKeys 805: 1–14. https://doi.org/10.3897/ zookeys.805.24834 Abstract Three new earthworm species are described from Sulawesi, Indonesia. Two belong to the genus Pithemera Sims & Easton, 1972, namely P. suwastikai Fahri, Amaliah & Atmowidi, sp. n. and P. tadulako Fahri, Amaliah & Atmowidi, sp. n. The new species, P. suwastikai sp. n. is distinguished by a medium size (135–165 mm long, 4.5–6.5 mm diameter), four pairs of spermathecal pores in 5/6/7/8/9, 7–12 setae between male pores, no genital markings, holandry, and simple intestinal caeca. Pithemera tadulako sp. -

A Case Study of the Exotic Peregrine Earthworm Morphospecies Pontoscolex Corethrurus Shabnam Taheri, Céline Pelosi, Lise Dupont
Harmful or useful? A case study of the exotic peregrine earthworm morphospecies Pontoscolex corethrurus Shabnam Taheri, Céline Pelosi, Lise Dupont To cite this version: Shabnam Taheri, Céline Pelosi, Lise Dupont. Harmful or useful? A case study of the exotic peregrine earthworm morphospecies Pontoscolex corethrurus. Soil Biology and Biochemistry, Elsevier, 2018, 116, pp.277-289. 10.1016/j.soilbio.2017.10.030. hal-01628085 HAL Id: hal-01628085 https://hal.archives-ouvertes.fr/hal-01628085 Submitted on 5 Jan 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Harmful or useful? A case study of the exotic peregrine earthworm MARK morphospecies Pontoscolex corethrurus ∗ ∗∗ S. Taheria, , C. Pelosib, L. Duponta, a Université Paris Est Créteil, Université Pierre et Marie Curie, CNRS, INRA, IRD, Université Paris-Diderot, Institut d’écologie et des Sciences de l'environnement de Paris (iEES-Paris), Créteil, France b UMR ECOSYS, INRA, AgroParisTech, Université Paris-Saclay, 78026 Versailles, France ABSTRACT Exotic peregrine earthworms are often considered to cause environmental harm and to have a negative impact on native species, but, as ecosystem engineers, they enhance soil physical properties. Pontoscolex corethrurus is by far the most studied morphospecies and is also the most widespread in tropical areas. -

F:\Zoos'p~1\2004\March2~1
CASE REPORT ZOOS' PRINT JOURNAL 19(3): 1394-1400 ILLUSTRATIONS OF EARTHWORMS OCCURING IN AND AROUND CHENNAI, INDIA V.I. Ramzan Begum 1 and Sultan Ahmed Ismail 2 1 Institute of Research in Soil Biology and Biotechnology, The New College, Chennai, Tamil Nadu 600014, India. 2 Corresponding author: Ecoscience Research Foundation, Plot 98, Baaz Nagar, 3/621 East Coast Road, Palavakkam, Chennai, Tamil Nadu 600041, India. 2 Email: [email protected] Abstract made to keep the description simple. Earthworms were collected from Chennai and its environs for taxonomic listing and to prepare a brief guide for Earthworms occur all over the world, but only rarely in deserts, identification by budding vermiculturists. Earthworm areas under constant snow and ice, mountain ranges and areas species were identified using existing taxonomic keys. almost entirely lacking in soil and vegetation (Edwards & Bohlen, Drawida scandens, Lampito mauritii, Perionyx 1996). excavatus, Perionyx sansibaricus, Octochaetona barnesi, Octochaetona pattoni, Octochaetona serrata, Earthworms belong to the Order Oligochaeta of the Phylum Octochaetona thurstoni, Dichogaster bolaui and Annelida. Oligochaetes are bilaterally symmetrical coelomate Eudrilus eugeniae have been recorded. D. bolaui was invertebrates with internal and external metameric segmentation the smallest while O. thurstoni was the largest species in throughout the body. Oligochaetes are often divided into two size observed. Fully mature adults of the common species convenient groups Microdrili and Megadrili. During the last collected over a period of time from sites in and around decade and half (Ismail, 1997) much taxonomic work on Indian Chennai are illustrated. Among the species, Eudrilus earthworms has been carried out by Julka (1975, 1976a,b, 1977, eugeniae does not occur naturally and are procured from 1978, 1979, 1981, 1983, 1993), Jamieson (1977) and Easton (1982). -
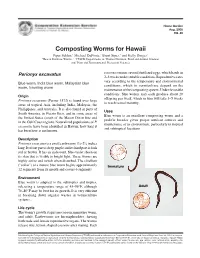
Composting Worms for Hawaii
Home Garden Aug. 2005 HG-46 Composting Worms for Hawaii Piper Selden,1 Michael DuPonte,2 Brent Sipes,3 and Kelly Dinges2 1Hawaii Rainbow Worms, 2, 3CTAHR Departments of 2Human Nutrition, Food and Animal Sciences and 3Plant and Environmental Protection Sciences Perionyx excavatus cocoon contains several fertilized eggs, which hatch in 2–3 weeks under suitable conditions. Reproductive rates Blue worm, India blue worm, Malaysian blue vary according to the temperature and environmental worm, traveling worm conditions, which in vermiculture depend on the maintenance of the composting system. Under favorable Origin conditions, blue worms may each produce about 20 offspring per week, which in turn will take 3–5 weeks Perionyx excavatus (Perrier 1872) is found over large to reach sexual maturity. areas of tropical Asia, including India, Malaysia, the Phillippines, and Australia. It is also found in parts of Uses South America, in Puerto Rico, and in some areas of Blue worm is an excellent composting worm and a the United States (south of the Mason Dixon line and prolific breeder given proper nutrient sources and in the Gulf Coast region). Naturalized populations of P. maintenance of its environment, particularly in tropical excavatus have been identified in Hawaii; how long it and subtropical locations. has been here is not known. Description 1 3 Perionyx excavatus is a small earthworm 1 ⁄4–2 ⁄4 inches long. Its front part is deep purple and its hind part is dark red or brown. It has an iridescent, blue-violet sheen on its skin that is visible in bright light. These worms are highly active and twitch when disturbed. -

Phylogenetic and Phenetic Systematics of The
195 PHYLOGENETICAND PHENETICSYSTEMATICS OF THE OPISTHOP0ROUSOLIGOCHAETA (ANNELIDA: CLITELLATA) B.G.M. Janieson Departnent of Zoology University of Queensland Brisbane, Australia 4067 Received September20, L977 ABSTMCT: The nethods of Hennig for deducing phylogeny have been adapted for computer and a phylogran has been constructed together with a stereo- phylogran utilizing principle coordinates, for alL farnilies of opisthopor- ous oligochaetes, that is, the Oligochaeta with the exception of the Lunbriculida and Tubificina. A phenogran based on the sane attributes conpares unfavourably with the phyLogralnsin establishing an acceptable classification., Hennigrs principle that sister-groups be given equal rank has not been followed for every group to avoid elevation of the more plesionorph, basal cLades to inacceptabl.y high ranks, the 0ligochaeta being retained as a Subclass of the class Clitellata. Three orders are recognized: the LumbricuLida and Tubificida, which were not conputed and the affinities of which require further investigation, and the Haplotaxida, computed. The Order Haplotaxida corresponds preciseLy with the Suborder Opisthopora of Michaelsen or the Sectio Diplotesticulata of Yanaguchi. Four suborders of the Haplotaxida are recognized, the Haplotaxina, Alluroidina, Monil.igastrina and Lunbricina. The Haplotaxina and Monili- gastrina retain each a single superfanily and fanily. The Alluroidina contains the superfamiJ.y All"uroidoidea with the fanilies Alluroididae and Syngenodrilidae. The Lurnbricina consists of five superfaniLies. -

Ecological Monitoring at Rare, 2020
Ecological Monitoring 2020 rare Charitable Research Reserve Prepared by: Jordan Wrobel Jenna Quinn 1 Acknowledgements Many thanks to Colleges and Institutes Canada (CICan) Career-Launcher Internships, funded by Natural Resource’s Canada’s Green Jobs- Science and Technology Internship Program, and Employment Ontario for providing essential funding for ecological monitoring at rare; without their support, this monitoring program and report would not have been possible. I would also like to thank rare staff for assistance with monitoring and support of intellectual and professional growth. Thank you to Caroline Reisiger and Sarah Cui for their much- appreciated assistance with fieldwork and to Dr. Justin Gaudon for your support with the statistical analyses. To rare’s committed volunteers: Jacqueline Haynes, Miriam Bauman, Emma Wegener, Hilary Irving, Bethany Wakefield, and Logan Mercier; thank you so much for your support with monitoring, these programs would not be as successful without you. I would like to thank all advocates of rare Charitable Research Reserve for helping to support rare’s vision and activities. The rare Charitable Research Reserve acknowledges and is grateful to all of the original stewards of the land in which rare resides, within the Haldimand Tract, spanning six miles on either side of the Grand River from source to mouth. Understanding that this land has been rich in diverse Indigenous presence since time immemorial, there are several Indigenous Nations that we would like to mention. We would like to honor and respect the sovereignty of both First Nations in our area: the Haudenosaunee Peoples of Six Nations of the Grand River and the Anishinaabe Peoples of Mississaugas of the New Credit First Nation. -

Biomolecular Approach to Oligochaete Taxonomy Dr
Dr. Jaya M et. al. / International Journal of New Technologies in Science and Engineering Vol. 2, Issue 6,Dec 2015, ISSN 2349-0780 Biomolecular Approach To Oligochaete Taxonomy Dr. Jaya. M 1* ,Dr. Aja. M2 and Dr. K. Vijayakumaran Nair3 1* Assistant Professor, Department of Zoology, Sree keralavarma College, Thrissur, Email: [email protected] 2Senior Research Fellow, Department of Zoology, University of Kerala, Kariavatom, Email: [email protected] 3Associate Professor, Department of Zoology, Mar Ivanios College, Thiruvananthapuram, Email: [email protected] ABSTRACT This paper comprises the molecular approach for the identification of earthworm along with the traditional taxonomic method. The mitochondrial CO 1 gene of the Pontoscolex corethrurus (Glossoscolecidae), Travoscolides chengannures, Amynthas corticis, Perionyx sansibaricus (Megascolecidae), Progizzardus varadiamensis and Glyphidrilus annandalei (Almidae) were sequenced. The cytochrome-c oxidase I (CO1) exhibited a unique barcode to a particular species. The further exploration of mitochondrial diversity in earthworms will lead to major improvements in our understanding of the evolutionary pathways and rates of the mitochondrial genome Key words: Barcoding, cytochrome c oxidase 1 (COI), 16S ribosomal DNA INTRODUCTION DNA barcoding is a taxonomic method that uses a short genetic marker in the DNA to identify an organism. It differs from molecular phylogeny in that the main goal is to identify an unknown sample in terms of a known classification [26]. DNA sequence can be used to identify different species, in the same way as the supermarket scanner uses the black stripes of the UPC barcode to identify the items. This database will rapidly link a specimen to a Binomial Linnaean name and through that link it will provide all available information and studies on the species. -

Amynthas) in the Northeast United States
Invertebrate Biology x(x): 1–14. © 2016 The Authors. Invertebrate Biology published by Wiley Periodicals, Inc. on behalf of American Microscopical Society. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made. DOI: 10.1111/ivb.12145 Phylogeographic analysis of invasive Asian earthworms (Amynthas) in the northeast United States Nancy Schult, Kelly Pittenger, Sam Davalos, and Damhnait McHugha Department of Biology, Colgate University, Hamilton, New York 13346, USA Abstract. Phylogeographic studies are useful in reconstructing the history of species inva- sions, and in some instances can elucidate cryptic diversity of invading taxa. This can help in predicting or managing the spread of invasive species. Among terrestrial invasive species in North America, earthworms can have profound ecological effects. We are familiar with the centuries-old invasions of European earthworms (Lumbricidae) and their impacts on nutrient cycling in soils. More recent invasions by Asian earthworms of the family Megascolecidae are less fully understood. We used data for two mitochondrial gene frag- ments, cytochrome oxidase I (COI) and 16S rRNA, to examine the relationships among populations of Asian earthworms in the megascolecid genus Amynthas in the northeast Uni- ted States. Recent reports have indicated that one species in particular, Amynthas agrestis, is having detrimental effects in mixed forest ecosystems, and we were interested in under- standing the invasion history for this species. We were surprised to discover three divergent mitochondrial lineages of Amynthas occurring sympatrically in upstate New York. -

Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems
Colby College Digital Commons @ Colby Honors Theses Student Research 2019 Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems Ella L. Maddi Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Environmental Sciences Commons, and the Soil Science Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Maddi, Ella L., "Asian Jumping Worm (Megascolecidae) Impacts on Physical and Biological Characteristics of Turfgrass Ecosystems" (2019). Honors Theses. Paper 965. https://digitalcommons.colby.edu/honorstheses/965 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented to the Faculty of the Department of Biology at Colby College in partial fulfillment of the requirements for the Degree of Bachelor of Arts with Honors By Ella Maddi Waterville, ME May 20, 2019 Asian Jumping Worm impacts (Megascolecidae) on Physical and Biological Characteristics of Turfgrass Ecosystems An Honors Thesis presented -

Earthworm Ecology from DARWIN to VERMICUL TURE for 1882
Earthworm Ecology FROM DARWIN TO VERMICUL TURE FOR 1882. MAN ·I~ ·BVT ·A·woR...JV\· Frontispiece Cartoon from Punch, December 6th, 1881 Earthworm Ecology FROM DARWIN TO VERMICUL TURE Edited by J. E. Satchell Institute of Terrestrial Ecology Merlewood Research Station Grange-over-Sands Cumbria, UK LONDON NEW YORK CHAPMAN AND HALL First published 1983 by Chapman and Hall Ltd I I New Fetter Lane, London EC4P 4EE Published in the USA by Chapman and Hall 733 Third Avenue, New York NY100I7 © 1983 Chapman and Hall Ltd Softcover reprint of the hardcover 1st edition 2007 University Press, Cambridge ISBN-13: 978-94-009-5967-5 e-ISBN-13: 978-94-009-5965-1 DOl: 10.1007/978-94-009-5965-1 All rights reserved. No part ofthis book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and record ing, or in any information storage and retrieval system, without permission in writing from the Publisher. British Library Cataloguing in Publication Data Earthworm ecology. I. Opisthopora I. Satchell, J. E. 595.I' 46 QL39 I. 04 Library of Congress Cataloging in Publication Data Main entry under title: Earthworm ecology. Bibliography: p. Includes index. I. Opisthopora-Ecology. 2. Earthworm culture. I. Satchell, John E. QL39I.A6E2 7 1983 Contents Preface Xl Contributors xiii DARWIN'S CONTRIBUTION TO EARTHWORM ECOLOGY I Darwin's Formation of Vegetable Mould- its philo sophical basis M. S. Ghilarov I 2 D~rwin on earthworms - the contemporary back ground and what the critics thought O. -

In the Phu Quoc Island, Vietnam, with Descriptions of Three New Species
ZooKeys 932: 1–25 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.932.50314 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research The megascolecid earthworms (Annelida, Oligochaeta, Megascolecidae) in the Phu Quoc island, Vietnam, with descriptions of three new species Tung T. Nguyen1, Dang H. Lam1, Binh T. K. Trinh2, Anh D. Nguyen3,4 1 Department of Biology, School of Education, Can Tho University, Can Tho City, Vietnam 2 Department of Applied Biology, Faculty of Agriculture and Rural Development, Kien Giang University, Kien Giang, Vietnam 3 Duy Tan University, 254, Nguyen Van Linh, Da Nang, Vietnam 4 Institute of Ecology and Biological Resourc- es, Vietnam Academy of Science and Technology, 18, Hoangquocviet Rd., Caugiay District, Hanoi, Vietnam Corresponding author: Anh D. Nguyen ([email protected]) Academic editor: Samuel James | Received 28 January 2020 | Accepted 13 April 2020 | Published 12 May 2020 http://zoobank.org/6C64E085-E11A-4AEE-85EE-3443E296C8DF Citation: Nguyen TT, Lam DH, Trinh BTK, Nguyen AD (2020) The megascolecid earthworms (Annelida, Oligochaeta, Megascolecidae) in the Phu Quoc island, Vietnam, with descriptions of three new species. ZooKeys 932: 1–25. https://doi.org/10.3897/zookeys.932.50314 Abstract The megascolecid earthworms of the Phu Quoc island are intensively investigated. Twelve species in three genera (Lampito Kinberg, 1867, Amynthas Kinberg, 1867, and Metaphire Sims & Easton, 1972) are re- corded. Of these, Metaphire doiphamon Bantaowong & Panha, 2016 is recorded for the first time in Vi- etnam, and three species are newly described, namely Amynthas catenatus sp. nov., A. phuquocensis sp. nov., and A. poropapillatus sp. -

(Annelida: Clitellata: Oligochaeta) Earthworms
etics & E en vo g lu t lo i y o h n a P r f y Journal of Phylogenetics & Perez-Losada et al., J Phylogen Evolution Biol 2015, 3:1 o B l i a o n l r o DOI: 10.4172/2329-9002.1000140 u g o y J Evolutionary Biology ISSN: 2329-9002 Research Article Open Access An Updated Multilocus Phylogeny of the Lumbricidae (Annelida: Clitellata: Oligochaeta) Earthworms Marcos Pérez-Losada1-3*, Jesse W Breinholt4, Manuel Aira5 and Jorge Domínguez5 1CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, 4485-661 Vairão, Portugal. 2Computational Biology Institute, George Washington University, Ashburn, VA 20147, USA 3Department of Invertebrate Zoology, US National Museum of Natural History, Smithsonian Institution, Washington, DC 20013, USA 4Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA 5Departamento de Ecoloxía e Bioloxía Animal, Universidade de Vigo, E-36310, Spain Abstract Lumbricidae earthworms dominate agricultural lands and often natural terrestrial ecosystems in temperate regions in Europe. They impact soil properties and nutrient cycling, shaping plant community composition and aboveground food webs. The simplicity of the earthworm body plan has hampered morphology-based classifications and taxonomy; hence current research on Lumbricidae systematic relies mostly on molecular data from multiple or single locus [e.g., cytochrome oxidase subunit I (COI) barcodes] to infer evolutionary relationships, validate taxonomic groups and/or identify species. Here we use multiple nuclear and mitochondrial gene regions (including COI) to generate updated maximum likelihood and Bayesian phylogenies of the family Lumbricidae. We then compare these trees to new COI trees to assess the performance of COI at inferring lumbricid inter-generic relationships.