Evidence-Based Clinical Practice Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pediatric Trauma Imaging from Head To
High yield pediatric imaging Pediatric Trauma and other Pediatric Emergencies Lynn Ansley Fordham, MD, FACR, FAIUM, FAAWR UNC School of Medicine Department of Radiology Division Chief Pediatric Radiology Overview • Discuss epidemiology of pediatric trauma • Discuss unique aspects of skeletal trauma and fractures in children • Look at a few example of fractures • Review other common pediatric emergencies • Some Pollev Pollev.com\lynnfordham • Lots cases Keywords for PACs case review • PTF • TUBES AND LINES • Pre call cases peds Who to page for peds 2am Thursday morning? Faculty call shift 5pm to 8 am, for midnight to 8am, use prior day schedule Etiology of Skeletal Trauma in Children Significance of pediatric injuries • injuries 40% deaths in 1-4 year olds • injuries 90% deaths in 5-19 year olds Causes of Mortality in Childhood • MVA most common in all age groups • pedestrian (vs. auto 5-9) • bicycle • firearms • fires • drowning/ near drowning Causes of Morbidity in Childhood • falls – most common cause of injury in children • non-fatal MVAs • bicycle related trauma • trampolines • near drowning • other non-fatal injuries Trends over time • Decrease in death rates due to unintentional injuries – Carseats – Bicycle helmets • Increase in homicide rate • Increase in suicide rate MVA common injuries • depends on impact and seatbelt status • spine – craniocervical junction – thoracolumbar spine • pelvis • extremities • sternum rare in children Pediatric Fractures • Fractures related to the physis (growth plate) – Use Salter Harris classification -

Natural Coral As a Biomaterial Revisited
American Journal of www.biomedgrid.com Biomedical Science & Research ISSN: 2642-1747 --------------------------------------------------------------------------------------------------------------------------------- Research Article Copy Right@ LH Yahia Natural Coral as a Biomaterial Revisited LH Yahia1*, G Bayade1 and Y Cirotteau2 1LIAB, Biomedical Engineering Institute, Polytechnique Montreal, Canada 2Neuilly Sur Seine, France *Corresponding author: LH Yahia, LIAB, Biomedical Engineering Institute, Polytechnique Montreal, Canada To Cite This Article: LH Yahia, G Bayade, Y Cirotteau. Natural Coral as a Biomaterial Revisited. Am J Biomed Sci & Res. 2021 - 13(6). AJBSR. MS.ID.001936. DOI: 10.34297/AJBSR.2021.13.001936. Received: July 07, 2021; Published: August 18, 2021 Abstract This paper first describes the state of the art of natural coral. The biocompatibility of different coral species has been reviewed and it has been consistently observed that apart from an initial transient inflammation, the coral shows no signs of intolerance in the short, medium, and long term. Immune rejection of coral implants was not found in any tissue examined. Other studies have shown that coral does not cause uncontrolled calcification of soft tissue and those implants placed under the periosteum are constantly resorbed and replaced by autogenous bone. The available porestudies size. show Thus, that it isthe hypothesized coral is not cytotoxic that a damaged and that bone it allows containing cell growth. both cancellous Thirdly, porosity and cortical and gradient bone can of be porosity better replacedin ceramics by isa graded/gradientexplained based on far from equilibrium thermodynamics. It is known that the bone cross-section from cancellous to cortical bone is non-uniform in porosity and in in vitro, animal, and clinical human studies. -

Avascular Necrosis As a Part of ‘Long COVID-19’ Sanjay R Agarwala,1 Mayank Vijayvargiya,2 Prashant Pandey2
Case report BMJ Case Rep: first published as 10.1136/bcr-2021-242101 on 2 July 2021. Downloaded from Avascular necrosis as a part of ‘long COVID-19’ Sanjay R Agarwala,1 Mayank Vijayvargiya,2 Prashant Pandey2 1Orthopaedics, PD Hinduja SUMMARY I or II, 92%–97% of the patients do not require National Hospital, Mumbai, ’Long COVID-19’ can affect different body systems. At surgery and can be managed with bisphosphonate Maharashtra, India present, avascular necrosis (AVN) as a sequalae of ’long therapy. Hence, it is crucial to diagnose AVN early 2Orthopaedics, PD Hinduja COVID-19’ has yet not been documented. By large- scale to decrease the morbidity and the requirement of National Hospital and Medical surgery. Research Centre, Mumbai, use of life- saving corticosteroids in COVID-19 cases, Here, we report three cases of symptomatic AVN Maharashtra, India we anticipate that there will be a resurgence of AVN cases. We report a series of three cases in which patients of the femoral head after being treated for COVID- Correspondence to developed AVN of the femoral head after being treated 19. This is the first case report of AVN as sequalae Dr Sanjay R Agarwala; for COVID-19 infection. The mean dose of prednisolone of ‘long COVID-19’. drsa2011@ gmail.com used in these cases was 758 mg (400–1250 mg), which is less than the mean cumulative dose of around 2000 CASE PRESENTATION Accepted 23 May 2021 mg steroid, documented in the literature as causative for Case 1 AVN. Patients were symptomatic and developed early A- 36- year old male patient was diagnosed with AVN presentation at a mean of 58 days after COVID-19 COVID-19 on 6 September 2020, for which the diagnosis as compared with the literature which shows patient was admitted in intensive care at another that it generally takes 6 months to 1 year to develop AVN hospital because of dropping saturation. -

Stage-Related Results in Treatment of Hip Osteonecrosis with Core-Decompression and Autologous Mesenchymal Stem Cells
Acta Orthop. Belg., 2015, 81, 406-412 ORIGINAL STUDY Stage-related results in treatment of hip osteonecrosis with core-decompression and autologous mesenchymal stem cells Pietro PERSIANI, Claudia DE CRISTO, Jole GRACI, Giovanni NOIA, Michele GURZÌ, Ciro VILLANI From Universitary Department of Anatomic, Histologic, Forensic and Locomotor Apparatus Sciences, Section of Locomotor Apparatus Sciences, Policlinico Umberto I, Sapienza University of Rome Our aim is to analyse the clinical outcome of a series thetic hip replacement. Therapy with glucocorti- of patients affected by avascular necrosis of the femo- coids and alcohol abuse are some of the main known ral head and treated with core-decompression tech- causative factors (21). Several pathophysiological nique and autologous stromal cells of the bone mechanisms were formulated about this pathology, marrow. including fat emboli, microvascular occlusion of We enrolled in our study 29 patients with 31 hips in epiphysis vessels and micro fracture of trabecular total affected by avascular necrosis of the femoral 13,18 head. bone ( ). An early diagnosis and a continuous The clinical and radiological outcome has been monitoring of patients with risk factors (corticoste- assessed through self-administered questionnaires roids therapy, alcoholism, or irradiated patients) are (HHS, VAS and SF12) X-ray and Magnetic Reso- very important in order to highlight the AVNFH nance. typical alteration as early as possible. Of all the examined hips, 25 showed a relief of the The treatment of osteonecrosis is one of the most symptoms and a resolution of the osteonecrosis, 11 of controversial subjects in the orthopaedic literature. these were at Stage I and 14 at Stage II. -

Patient Guide for a Hip Replacement
Day of surgery: ____________________ Expected discharge home: ____________________ Surgeon: ____________________ Phone number: ____________________ Bring this guide with you to the hospital on the day of your surgery 4100492 (17-10) 2 Introduction This information guide will help you understand what is involved in hip arthroplasty (hip replacement). We hope the information in this booklet will help you prepare for your surgery •••••••••••••••••••••••••••••••••••••••••••••••••••••• About Hôpital Montfort Hôpital Montfort is a Francophone academic health care institution that provides quality care in both official languages and works with its partners to improve the health of communities. Montfort strives for excellence and wants to be a hospital of choice for its personalized patient care, its workplace, teaching and research. Our daily actions are guided by the values of compassion, commitment, excellence and respect. WARNING This guide does not replace the advice of your care provider. Please consult your care provider to assess if the information presented in this guide applies to your situation. The content of this guide was prepared by Vancouver Coastal Health and adapted by Hôpital Montfort. 3 Your Health Care: Be Involved! • Be involved in your health care. Speak up if you have questions or concerns about your care. • Tell a member of your health care team about your past illnesses and your current health condition. • Tell your health team if you have any food or medication allergies. • Make sure you know what to do when you leave the hospital. •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Your Interprofessional Plan of Care • Your hospital admission for your total hip replacement will follow an “interprofessional plan of care” more commonly called a "Clinical Pathway". -

Clinical Management of Severe Acute Respiratory Infections When Novel Coronavirus Is Suspected: What to Do and What Not to Do
INTERIM GUIDANCE DOCUMENT Clinical management of severe acute respiratory infections when novel coronavirus is suspected: What to do and what not to do Introduction 2 Section 1. Early recognition and management 3 Section 2. Management of severe respiratory distress, hypoxemia and ARDS 6 Section 3. Management of septic shock 8 Section 4. Prevention of complications 9 References 10 Acknowledgements 12 Introduction The emergence of novel coronavirus in 2012 (see http://www.who.int/csr/disease/coronavirus_infections/en/index. html for the latest updates) has presented challenges for clinical management. Pneumonia has been the most common clinical presentation; five patients developed Acute Respira- tory Distress Syndrome (ARDS). Renal failure, pericarditis and disseminated intravascular coagulation (DIC) have also occurred. Our knowledge of the clinical features of coronavirus infection is limited and no virus-specific preven- tion or treatment (e.g. vaccine or antiviral drugs) is available. Thus, this interim guidance document aims to help clinicians with supportive management of patients who have acute respiratory failure and septic shock as a consequence of severe infection. Because other complications have been seen (renal failure, pericarditis, DIC, as above) clinicians should monitor for the development of these and other complications of severe infection and treat them according to local management guidelines. As all confirmed cases reported to date have occurred in adults, this document focuses on the care of adolescents and adults. Paediatric considerations will be added later. This document will be updated as more information becomes available and after the revised Surviving Sepsis Campaign Guidelines are published later this year (1). This document is for clinicians taking care of critically ill patients with severe acute respiratory infec- tion (SARI). -

Acute Chest Syndrome, Avascular Necrosis of Femur, and Pulmonary Embolism All at Once: an Unexpected Encounter in the First-Ever Admission of a Sickle Cell Patient
Open Access Case Report DOI: 10.7759/cureus.17656 Acute Chest Syndrome, Avascular Necrosis of Femur, and Pulmonary Embolism All at Once: An Unexpected Encounter in the First-Ever Admission of a Sickle Cell Patient Akhilesh Annadatha 1 , Dhruv Talwar 1 , Sourya Acharya 1 , Sunil Kumar 1 , Vivek Lahane 1 1. Department of Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Deemed University), Wardha, IND Corresponding author: Dhruv Talwar, [email protected] Abstract Acute chest syndrome (ACS) is defined as the radiological appearance of pulmonary infiltrates with fever or respiratory symptoms like chest pain, breathlessness, and cough in a patient with sickle cell disease (SCD). It is also a very common cause of mortality in sickle cell patients, if not identified in early stages and treated aggressively. Radiological image is similar to bacterial pneumonia, so sickle cell disease with a radiological picture similar to pneumonia and associated respiratory symptoms is known as acute chest syndrome. Pneumonia and infarction have been implicated in pathogenesis. The reason for the appearance of acute chest syndrome in patients with SCD is not established but some triggers like sepsis, presence of vaso- occlusive crises have been noted. When there is a block in the blood supply to the bone, patients with sickle cell disease may also develop avascular necrosis of the neck of the femur causing narrowing of joint and collapse of the bone. Patients with sickle cell disease have a baseline hypercoagulable state thereby predisposing the patient to develop deep vein thrombosis and pulmonary embolism. Here, we present a case of a 25-year-old SCD patient with a fairly stable course of the disease. -

Symptomatic Middle Ear and Cranial Sinus Barotraumas As a Complication of Hyperbaric Oxygen Treatment
İst Tıp Fak Derg 2016; 79: 4 KLİNİK ARAŞTIRMA / CLINICAL RESEARCH J Ist Faculty Med 2016; 79: 4 http://dergipark.ulakbim.gov.tr/iuitfd http://www.journals.istanbul.edu.tr/iuitfd SYMPTOMATIC MIDDLE EAR AND CRANIAL SINUS BAROTRAUMAS AS A COMPLICATION OF HYPERBARIC OXYGEN TREATMENT HİPERBARİK OKSİJEN TEDAVİSİ KOMPLİKASYONU: SEMPTOMATİK ORTA KULAK VE KRANYAL SİNUS BAROTRAVMASI Bengüsu MİRASOĞLU*, Aslıcan ÇAKKALKURT*, Maide ÇİMŞİT* ABSTRACT Objective: Hyperbaric oxygen therapy (HBOT) is applied for various diseases. It is generally considered safe but has some benign complications and adverse effects. The most common complication is middle ear barotrauma. The aim of this study was to collect data about middle ear and cranial sinus barotraumas in our department and to evaluate factors affecting the occurrence of barotrauma. Material and methods: Files of patients who had undergone hyperbaric oxygen therapy between June 1st, 2004, and April 30th, 2012, and HBOT log books for the same period were searched for barotraumas. Patients who were intubated and unconscious were excluded. Data about demographics and medical history of conscious patients with barotrauma (BT) were collected and evaluated retrospectively. Results: It was found that over eight years and 23,645 sessions, 39 of a total 896 patients had BT; thus, the general BT incidence of our department was 4.4%. The barotrauma incidence was significantly less in the multiplace chamber (3.1% vs. 8.7%) where a health professional attended the therapies. Most barotraumas were seen during early sessions and were generally mild. A significant accumulation according to treatment indications was not determined. Conclusion: It was thought that the low barotrauma incidence was related to the slow compression rate as well as training patients thoroughly and monitoring them carefully. -
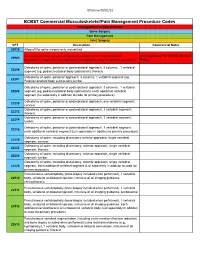
Commercial MSK Procedure Code List Effective 09.01.21.Xlsx
Effective 09/01/21 BCBST Commercial Musculoskeletal/Pain Management Procedure Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery CPT Description Commercial Notes 20930 Allograft for spine surgery only morselized Computer-assisted surgical navigational procedure for musculoskeletal Investigational Per BCBST Medical 20985 procedures, image-less (List separately in addition to code for primary procedure) Policy Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22206 segment (eg, pedicle/vertebral body subtraction); thoracic Osteotomy of spine, posterior approach, 3 columns, 1 vertebral segment (eg. 22207 Pedicle/vertebral body subtraction);lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral 22208 segment (eg, pedicle/vertebral body subtraction); each additional vertebral segment (list separately in addition to code for primary procedure) Osteotomy of spine, posterior or posterolateral approach, one vertebral segment; 22210 cervical Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22212 thoracic Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22214 lumbar Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; 22216 each additional vertebral segment (List separately in addition to primary procedure) Osteotomy of spine, including discectomy anterior approach, single vertebral 22220 segment; cervical Osteotomy of spine, including discectomy, anterior approach, single -

Hip Impingement and Osteoarthritis. Hip Arthroscopy, Resurfacing, Or Replacement: Is It Time for Surgery?
Hip Impingement and Osteoarthritis. Hip Arthroscopy, Resurfacing, or Replacement: Is it Time for Surgery? Bryan Whitfield, MD [email protected] Hip Operations • Background of hip diagnoses • Treatment of hip pathology • Surgery candidates • Time for surgery? • Cases Intra-articular hip pain - diagnoses • FAI (femoroacetabular impingement) / acetabular labrum tear • Neck of the femur and rim of the socket run into each other • Tear the labrum. • Articular cartilage damage possible • Labrum: fluid management, stability, and seal of the joint • Osteoarthritis • Socket and ball both coated in a layer of cartilage • Arthritis is the wearing away, loss of or damage to the cartilage coating (articular cartilage) Intra-articular hip pain - diagnoses • FAI (femoroacetabular impingement) / acetabular labrum tear • Neck of the femur and rim of the socket run into each other • Tear the labrum. • Articular cartilage damage possible • Labrum: fluid management, stability, and seal of the joint • Osteoarthritis • Socket and ball both coated in a layer of cartilage • Arthritis is the wearing away, loss of or damage to the cartilage coating (articular cartilage) Intra-articular hip pain - diagnoses • FAI (femoroacetabular impingement) / acetabular labrum tear • Neck of the femur and rim of the socket run into each other • Tear the labrum. • Articular cartilage damage possible • Labrum: fluid management, stability, and seal of the joint • Osteoarthritis • Socket and ball both coated in a layer of cartilage • Arthritis is the wearing away, loss -

Dr. Mark Dolan Specializes in Hip and Knee Replacement Surgery, Hip Resurfacing, and Hip Arthroscopy/Hip Preservation Surgery
Dr. Mark Dolan specializes in hip and knee replacement surgery, hip resurfacing, and hip arthroscopy/hip preservation surgery. Dr. Dolan is originally from South Bend, Indiana and a graduate of the University of Notre Dame. He attended Indiana University School of Medicine before moving to Chicago to continue his education in orthopaedic surgery as a resident in Northwestern University Feinberg School of Medicine’s Department of Orthopaedic Surgery. After residency he completed a fellowship in Adult Reconstruction and Joint Replacement Surgery at the Hospital for Special Surgery in New York where he gained advanced training in hip and knee replacement surgery, hip resurfacing, and revision total joint surgery. Following this year long fellowship, he stayed at the Hospital for Special Surgery to obtain additional experience in providing care for young adults with hip pain. This included additional study in hip arthroscopy and hip preservation surgery. Dr. Dolan has a specific interest in hip disorders and strives to provide comprehensive care to patients suffering from hip pain. This includes non-operative treatment as well as total joint replacement, hip resurfacing, and arthroscopic hip surgery (athletic hip injuries, hip joint preservation surgery, femoracetabular impingement (FAI), and acetabular labral injuries). Dr. Dolan utilizes this wide array of treatment options to best serve his patients, working with each patient to develop a focused and individualized treatment plan. As a former varsity soccer player at Notre Dame and an active runner and skier, Dr. Dolan appreciates the importance of maintaining an active lifestyle. His goal is to relieve your pain and improve your mobility, thereby allowing you to return to an active life. -

V.R.K.V. Prasad Gourineni
V.R.K.V. Prasad Gourineni PERSONAL DATA 1401 Burr Oak Road, #402B, Hinsdale, IL 60521. Tel 630-789-9223 [email protected] Citizenship: India, U.S. Permanent resident. CURRENT APPOINTMENTS Clinical Associate Professor in Orthopaedics 2014 – Present University of Illinois, Chicago Head, Division of Pediatric Orthopaedics December ‘06 – Present Advocate Children’s Hospital, Oak Lawn Orthopaedic surgery private practice August ’98 - Present Pediatric & Young Adult Orthopaedics December ’06 - Present EDUCATION Medical School Aug 78 - Jan 85 Andhra Medical College, India GRADUATE MEDICAL EDUCATION Fellowship in Pediatric Orthopaedics August 97 - July 98 Texas Scottish Rite Hospital, Dallas Residency in Orthopaedic surgery June 92 - June 97 Northwestern University, Chicago OITE ‘96 - 98%ile, ‘95 - 100%ile, ‘94 - 96%ile, ‘93 - 99%ile Transitional Internship June 91 - June 92 St. Francis Hospital, Evanston Residency in Orthopaedic surgery, India June 86 - July 89 Mangalore University RESEARCH TRAINING Research in Orthopaedics & Biomechanics Loyola University Oct 90 – May 91 BOARD CERTIFICATION American Board of Orthopaedic Surgeons Part I - 99%ile, July 97 Part II – July 2001 Recertification July 2010 National Board of Medical Examiners I - 690 / 98%ile, June 90 (ECFMG certification 447-777-4) II - 605 / 89%ile, Sept 90 III - FLEX, Dec 90 1 MEDICINE LICENSURE Illinois Department of Professional Regulation Indiana Medical License Indian Medical Council HONORS & AWARDS Exceptional Health Outcomes Advocate Physician Partners 2009-12. Anatomy Prize, 93 & 95 Northwestern University, Chicago. Anatomy Prize, 94 & 96 honorably excluded from competition. Resident of the year, 1994 Lutheran General Hospital, Park Ridge. Multiple Spirit awards, 1994 Lutheran General Hospital, Park Ridge. First Place Award, Nov ‘92 Resident research paper, American (Co-author) Society for Surgery of the Hand.