Grossmanite, Cati3+Alsio6, a New Pyroxene from the Allende Meteorite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Addibischoffite, Ca2al6al6o20, a New Calcium Aluminate Mineral from The
1 Revision 3 2 Addibischoffite, Ca2Al6Al6O20, a new calcium aluminate mineral from 3 the Acfer 214 CH carbonaceous chondrite: A new refractory phase from 4 the solar nebula 5 Chi Ma1,*, Alexander N. Krot2, Kazuhide Nagashima2 6 1Division of Geological and Planetary Sciences, California Institute of Technology, 7 Pasadena, California 91125, USA 8 2Hawai‘i Institute of Geophysics and Planetology, University of Hawai‘i at Mānoa, 9 Honolulu, Hawai‘i 96822, USA 10 11 ABSTRACT 12 Addibischoffite (IMA 2015-006), Ca2Al6Al6O20, is a new calcium aluminate mineral 13 that occurs with hibonite, perovskite, kushiroite, Ti-kushiroite, spinel, melilite, 14 anorthite and FeNi-metal in the core of a Ca-Al-rich inclusion (CAI) in the Acfer 15 214 CH3 carbonaceous chondrite. The mean chemical composition of type 16 addibischoffite by electron probe microanalysis is (wt%) Al2O3 44.63, CaO 15.36, 17 SiO2 14.62, V2O3 10.64, MgO 9.13, Ti2O3 4.70, FeO 0.46, total 99.55, giving rise to 18 an empirical formula of 3+ 3+ 2+ 19 (Ca2.00)(Al2.55Mg1.73V 1.08Ti 0.50Ca0.09Fe 0.05)Σ6.01(Al4.14Si1.86)O20. The general 20 formula is Ca2(Al,Mg,V,Ti)6(Al,Si)6O20. The end-member formula is Ca2Al6Al6O20. 21 Addibischoffite has the P1 aenigmatite structure with a = 10.367 Å, b = 10.756 Å, c 22 = 8.895 Å, α = 106.0°, β = 96.0°, γ = 124.7°, V = 739.7 Å3, and Z = 2, as revealed by 23 electron back-scatter diffraction. The calculated density using the measured 24 composition is 3.41 g/cm3. -

Chance and Necessity in the Mineral Diversity of Terrestrial Planets
295 The Canadian Mineralogist Vol. 53, pp. 295-324 (2015) DOI: 10.3749/canmin.1400086 MINERAL ECOLOGY: CHANCE AND NECESSITY IN THE MINERAL DIVERSITY OF TERRESTRIAL PLANETS § ROBERT M. HAZEN Geophysical Laboratory, Carnegie Institution of Washington, 5251 Broad Branch Road NW, Washington, DC 20015, U.S.A. EDWARD S. GREW School of Earth and Climate Sciences, University of Maine, Orono, Maine 04469, U.S.A. ROBERT T. DOWNS AND JOSHUA GOLDEN Department of Geosciences, University of Arizona, 1040 E. 4th Street, Tucson, Arizona 85721-0077, U.S.A. GRETHE HYSTAD Department of Mathematics, University of Arizona, 617 N. Santa Rita Ave., Tucson, Arizona 85721-0089, U.S.A. ABSTRACT Four factors contribute to the roles played by chance and necessity in determining mineral distribution and diversity at or near the surfaces of terrestrial planets: (1) crystal chemical characteristics; (2) mineral stability ranges; (3) the probability of occurrence for rare minerals; and (4) stellar and planetary stoichiometries in extrasolar systems. The most abundant elements generally have the largest numbers of mineral species, as modeled by relationships for Earth’s upper continental crust (E) and the Moon (M), respectively: 2 LogðNEÞ¼0:22 LogðCEÞþ1:70 ðR ¼ 0:34Þð4861 minerals; 72 elementsÞ 2 LogðNMÞ¼0:19 LogðCMÞþ0:23 ðR ¼ 0:68Þð63 minerals; 24 elementsÞ; where C is an element’s abundance in ppm and N is the number of mineral species in which that element is essential. Several elements that plot significantly below the trend for Earth’s upper continental crust (e.g., Ga, Hf, and Rb) mimic other more abundant elements and thus are less likely to form their own species. -

An Evolutionary System of Mineralogy, Part II: Interstellar and Solar Nebula Primary Condensation Mineralogy (> 4.565
This is a preprint, the final version is subject to change, of the American Mineralogist (MSA) Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2020-7447 1 REVISION #2—07 May 2020—American Mineralogist 2 3 An evolutionary system of mineralogy, part II: Interstellar and solar 4 nebula primary condensation mineralogy (> 4.565 Ga) 5 1 1,* 6 SHAUNNA M. MORRISON AND ROBERT M. HAZEN 7 1Earth and Planets Laboratory, Carnegie Institution for Science, 8 5251 Broad Branch Road NW, Washington DC 20015, U. S. A. 9 10 11 ABSTRACT 12 The evolutionary system of mineralogy relies on varied physical and chemical attributes, 13 including trace elements, isotopes, solid and fluid inclusions, and other information-rich 14 characteristics, to understand processes of mineral formation and to place natural condensed 15 phases in the deep-time context of planetary evolution. Part I of this system reviewed the earliest 16 refractory phases that condense at T > 1000 K within the turbulent expanding and cooling 17 atmospheres of highly evolved stars. Part II considers the subsequent formation of primary 18 crystalline and amorphous phases by condensation in three distinct mineral-forming environments, 19 each of which increased mineralogical diversity and distribution prior to the accretion of 20 planetesimals > 4.5 billion years ago: 21 1) Interstellar molecular solids: Varied crystalline and amorphous molecular solids containing 22 primarily H, C, O, and N are observed to condense in cold, dense molecular clouds in the 23 interstellar medium (10 < T < 20 K; P < 10-13 atm). With the possible exception of some 24 nano-scale organic condensates preserved in carbonaceous meteorites, the existence of Always consult and cite the final, published document. -

Mineralogy of Silicate-Natrophosphate Immiscible † Inclusion in Elga IIE Iron Meteorite
minerals Article Mineralogy of Silicate-Natrophosphate Immiscible y Inclusion in Elga IIE Iron Meteorite Victor V. Sharygin 1,2 1 V.S. Sobolev Institute of Geology and Mineralogy, Siberian Branch of the RAS, 3 Acad. Koptyuga pr., 630090 Novosibirsk, Russia; [email protected] 2 ExtraTerra Consortium, Institute of Physics and Technology, Ural Federal University, 19 Mira str., 620002 Ekaterinburg, Russia The paper was presented at the 81st Annual Meeting of the Meteoritical Society in Moscow, Russia, y 22–27 July 2018. Received: 7 April 2020; Accepted: 11 May 2020; Published: 13 May 2020 Abstract: Rare type of silicate inclusions found in the Elga iron meteorite (group IIE) has a very specific mineral composition and shows silicate ( 90%)–natrophosphate ( 10%) liquid ≈ ≈ immiscibility due to meniscus-like isolation of Na-Ca-Mg-Fe phosphates. The 3 mm wide immiscible inclusion has been first studied in detail using optical microscopy, scanning electron microscopy, electron microprobe analysis and Raman spectroscopy. The silicate part of the inclusion contains fine-grained quartz-feldspar aggregate and mafic minerals. The relationships of feldspars indicate solid decay of initially homogenous K-Na-feldspar into albite and K-feldspar with decreasing of temperature. Some mafic minerals in the silicate part are exotic in composition: the dominant phase is an obertiite-subgroup oxyamphibole (amphibole supergroup), varying from ferri-obertiite 3+ 2+ NaNa2Mg3Fe Ti[Si8O22]O2 to hypothetical NaNa2Mg3Fe 0.5Ti1.5[Si8O22]O2; minor phases are the aenigmatite-subgroup mineral (sapphirine supergroup) with composition close to median value 2+ of the Na2Fe 5TiSi6O18O2-Na2Mg5TiSi6O18O2 join, orthopyroxene (enstatite), clinopyroxene of the diopside Ca(Mg,Fe)Si2O6–kosmochlor NaCrSi2O6-Na(Mg,Fe)0.5Ti0.5Si2O6 series and chromite. -

XANES and Mg Isotopic Analyses of Spinels in Caalrich Inclusions
Meteoritics & Planetary Science 48, Nr 10, 2015–2043 (2013) doi: 10.1111/maps.12216 XANES and Mg isotopic analyses of spinels in Ca-Al-rich inclusions: Evidence for formation under oxidizing conditions J. M. PAQUE1*, S. R. SUTTON2, S. B. SIMON2, J. R. BECKETT1, D. S. BURNETT1, 2,3 4 4 5–8 L. GROSSMAN , H. YURIMOTO , S. ITOH , and H. C. CONNOLLY JR. 1Division of Geological and Planetary Sciences, Caltech, Pasadena, California 91125, USA 2Department of Geophysical Sciences, The University of Chicago, Chicago, Illinois 60637, USA 3Enrico Fermi Institute, The University of Chicago, 5640 S Ellis Ave., Chicago, Illinois 60637, USA 4Department of Natural History Science, Hokkaido University, Sapporo, Hokkaido, Japan 5Department of Physical Sciences, Kingsborough Community College of the City University of New York, 2001 Oriental Boulevard, Brooklyn, New York 11235, USA 6Department of Earth and Environmental Sciences, The Graduate Center of the City University of New York, New York, New York 10016, USA 7Department of Earth and Planetary Sciences, American Museum of Natural History, New York, New York 10024, USA 8Lunar and Planetary Laboratory, University of Arizona, Tucson, Arizona 85721, USA *Corresponding author. E-mail: [email protected] (Received 11 March 2013; revision accepted 12 September 2013) Abstract–Ti valence measurements in MgAl2O4 spinel from calcium-aluminum-rich inclusions (CAIs) by X-ray absorption near-edge structure (XANES) spectroscopy show that many spinels have predominantly tetravalent Ti, regardless of host phases. The average spinel in Allende type B1 inclusion TS34 has 87% Ti+4. Most spinels in fluffy type A (FTA) inclusions also have high Ti valence. -

Meteorite Mineralogy Alan Rubin , Chi Ma Index More Information
Cambridge University Press 978-1-108-48452-7 — Meteorite Mineralogy Alan Rubin , Chi Ma Index More Information Index 2I/Borisov, 104, See interstellar interloper alabandite, 70, 96, 115, 142–143, 151, 170, 174, 177, 181, 187, 189, 306 Abbott. See meteorite Alais. See meteorite Abee. See meteorite Albareto. See meteorite Acapulco. See meteorite Albin. See meteorite acapulcoites, 107, 173, 179, 291, 303, Al-Biruni, 3 309, 314 albite, 68, 70, 72, 76, 78, 87, 92, 98, 136–137, 139, accretion, 238, 260, 292, 347, 365 144, 152, 155, 157–158, 162, 171, 175, 177–178, acetylene, 230 189–190, 200, 205–206, 226, 243, 255–257, 261, Acfer 059. See meteorite 272, 279, 295, 306, 309, 347 Acfer 094. See meteorite albite twinning, 68 Acfer 097. See meteorite Aldrin, Buzz, 330 achondrites, 101, 106–108, 150, 171, 175, 178–179, Aletai. See meteorite 182, 226, 253, 283, 291, 294, 303, 309–310, 318, ALH 77307. See meteorite 350, 368, 374 ALH 78091. See meteorite acute bisectrix, 90 ALH 78113. See meteorite adamite, 83 ALH 81005. See meteorite addibischoffite, 116, 167 ALH 82130. See meteorite Adelaide. See meteorite ALH 83009. See meteorite Adhi Kot. See meteorite ALH 83014. See meteorite Admire. See meteorite ALH 83015. See meteorite adrianite, 117, 134, 167, 268 ALH 83108. See meteorite aerogel, 234 ALH 84001. See meteorite Aeschylus, 6 ALH 84028. See meteorite agate, 2 ALH 85085. See meteorite AGB stars. See asymptotic giant branch stars ALH 85151. See meteorite agglutinate, 201, 212, 224, 279, 301–302, 308 ALHA76004. See meteorite Agpalilik. See Cape York ALHA77005. -

Hutcheonite, Ca3ti2(Sial2)O12, a New Garnet Mineral from the Allende
1 Revision 2 2 Hutcheonite, Ca3Ti2(SiAl2)O12, a new garnet mineral from the Allende 3 meteorite: An alteration phase in a Ca-Al-rich inclusion 4 Chi Ma1* and Alexander N. Krot2 5 1Division of Geological and Planetary Sciences, California Institute of Technology, 6 Pasadena, California 91125, USA 7 2Hawai‘i Institute of Geophysics and Planetology, University of Hawai‘i at Mānoa, 8 Honolulu, Hawai‘i 96822, USA 9 10 ABSTRACT 11 Hutcheonite (IMA 2013-029), Ca3Ti2(SiAl2)O12, is a new garnet mineral that occurs 12 with monticellite, grossular and wadalite in secondary alteration areas along some 13 cracks between primary melilite, spinel and Ti,Al-diopside in a Type B1 FUN 14 (Fractionation and Unidentified Nuclear effects) Ca-Al-rich inclusion (CAI) Egg-3 15 from the Allende CV (Vigarano type) carbonaceous chondrite. The mean chemical 16 composition of type hutcheonite by electron probe microanalysis is (wt%) CaO 34.6, 17 TiO2 25.3, SiO2 20.9, Al2O3 15.7, MgO 2.1, FeO 0.7, V2O3 0.5, total 99.8, giving rise 4+ 2+ 3+ 18 to an empirical formula of Ca2.99(Ti 1.53Mg0.25Al0.17Fe 0.05V 0.03)(Si1.68Al1.32)O12. 19 The end-member formula is Ca3Ti2(SiAl2)O12. Hutcheonite has the Ia-3d garnet 20 structure with a = 11.843 Å, V = 1661.06 Å3, and Z = 8, as revealed by electron 21 back-scatter diffraction. The calculated density using the measured composition is 22 3.86 g/cm3. Hutcheonite is a new secondary phase in Allende, apparently formed by 23 iron-alkali-halogen metasomatic alteration of the primary CAI phases like melilite, 24 perovskite, and Ti,Al-diopside on the CV chondrite parent asteroid. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -
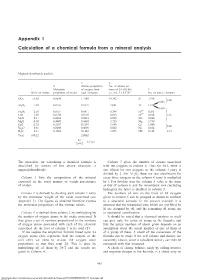
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Microstructural Constraints on the Formational and Thermal Histories of Refractory Inclusions in Co3 Chondrites." (2014)
University of New Mexico UNM Digital Repository Earth and Planetary Sciences ETDs Electronic Theses and Dissertations 5-1-2014 MICROSTRUCTURAL ONC STRAINTS ON THE FORMATIONAL AND THERMAL HISTORIES OF REFRACTORY INCLUSIONS IN CO3 CHONDRITES Jangmi Han Follow this and additional works at: https://digitalrepository.unm.edu/eps_etds Recommended Citation Han, Jangmi. "MICROSTRUCTURAL CONSTRAINTS ON THE FORMATIONAL AND THERMAL HISTORIES OF REFRACTORY INCLUSIONS IN CO3 CHONDRITES." (2014). https://digitalrepository.unm.edu/eps_etds/34 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at UNM Digital Repository. It has been accepted for inclusion in Earth and Planetary Sciences ETDs by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Jangmi Han Candidate Earth and Planetary Sciences Department This dissertation is approved, and it is acceptable in quality and form for publication: Approved by the Dissertation Committee: Dr. Adrian J. Brearley, Chairperson Dr. Rhian H. Jones Dr. Zachary D. Sharp Dr. Charles K. Shearer, Jr. Dr. Lindsay P. Keller i MICROSTRUCTURAL CONSTRAINTS ON THE FORMATIONAL AND THERMAL HISTORIES OF REFRACTORY INCLUSIONS IN CO3 CHONDRITES BY JANGMI HAN B.S., Earth Science Education, Seoul National University, 2007 M.S., Earth Science Education, Seoul National University, 2009 DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Earth and Planetary Sciences The University of New Mexico Albuquerque, New Mexico May, 2014 ii © 2014 Jangmi Han iii ACKNOWLEDGEMENTS This study was supported by NASA Cosmochemistry Program through grants NNG06GG37G and NNX12AH59G to Dr. Adrian J. Brearley (P.I). -

New Mineral Names*,†
American Mineralogist, Volume 105, pages 1920–1925, 2020 New Mineral Names*,† Dmitriy I. Belakovskiy1, Yulia Uvarova2, and Fernando Cámara3 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2CSIRO Mineral Resources, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151, Australia 3Dipartimento di Scienze della Terra “Ardito Desio”, Università degli Studi di Milano, Via Mangiagalli, 34, 20133 Milano, Italy In this issue This New Mineral Names has entries for 13 new species, including falottaite, meieranite and high-pressure minerals found in meteorites, terrestrial impact rocks, and as inclusions in diamonds: hemleyite, hiroseite, ice-VII, kaitianite, maohokite, proxidecagonite, riesite, rubinite, uakitite, wangdaodeite, and zagamiite. Falottaite* ment was not performed. The mineral was named for its type locality. Type material (now in the form of the lindbergite pseudomorph after S. Graeser and W Gabriel (2016) Falottait (MnC2O4·3H2O)—ein neues Oxalat-Mineral aus den Schweizer Alpen. Schweizer Strahler, 50(3), falottaite crystals) is deposited in the Natural History Museum Basel, 20–27 (in German and French). Basel, Switzerland. D.B. References cited Falottaite (IMA 2013-044), MnC2O4·3H2O, orthorhombic, is a new mineral discovered in abandoned manganese mines of Falotta and JCPDS International Center for Diffraction Data (1988) Swarthmore, Pennsylvania. Card # 32-648. Parsettens and Tinzen in the Oberhalbstein region, Canton Grisons, Switzerland. The mines were exploited in the past centuries up to the Hemleyite* World War II. The manganese ores occur as lenses in radiolaritic rocks L. Bindi, M. Chen, and X. Xie (2017) Discovery of the Fe-analogue of of the large Oberhalbstein ophiolithic zone. -

Planetesimal Differentiation and Impact Mineralization
This is the peer-reviewed, final accepted version for American Mineralogist, published by the Mineralogical Society of America. The published version is subject to change. Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2021-7632. http://www.minsocam.org/ 1 #7632—REVISION #2—03 August 2020—submitted to American Mineralogist 2 3 An evolutionary system of mineralogy, Part IV: Planetesimal 4 differentiation and impact mineralization (4566 to 4560 Ma) 1 1,* 5 SHAUNNA M. MORRISON AND ROBERT M. HAZEN 1 6 Earth and Planets Laboratory, Carnegie Institution for Science, 7 5251 Broad Branch Road NW, Washington D.C. 20015, U. S. A. 8 ABSTRACT 9 The fourth installment of the evolutionary system of mineralogy considers two stages of 10 planetesimal mineralogy that occurred early in the history of the solar nebula, commencing by 11 4.566 Ga and lasting for at least 5 million years: (1) primary igneous minerals derived from 12 planetesimal melting and differentiation into core, mantle, and basaltic components; and (2) 13 impact mineralization resulting in shock-induced deformation, brecciation, melting, and high- 14 pressure phase transformations. 15 We tabulate 90 igneous differentiated asteroidal minerals, including the earliest known 16 occurrences of minerals with Ba, Cl, Cu, F, and V as essential elements, as well as the first 17 appearances of numerous phosphates, quartz, zircon, and amphibole group minerals. We also 18 record 40 minerals formed through high-pressure impact alteration, commencing with the period 19 of asteroid accretion and differentiation. These stages of mineral evolution thus mark the first 20 time that high pressures, both static and dynamic, played a significant role in mineral 21 paragenesis.