Sensitive Cilia – Eyelashes in Health and Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Trusted Child Health Care Since 1958 Thank You for Voting Us As One of Des Moines’ Favorite!
DES MOINES PARENTMAGAZINE www.DesMoinesParent.com august 2017 Back to SCHOOL!Healthy habit ideas you can use for the new school year The results are in! DES MOINES PARENTMAGAZINE Readers Choice AWARDS CONTEST PAGES 16-22 Soccer progams Kids eat free! Make homework fun PAGE 4 PAGE 6 PAGE 24 2 DES MOINES PARENT MAGAZINE AUGUST 2017 WWW.DESMOINESPARENT.COM Back to school By Erin Huiatt This summer has flown by. If your children are not back in school President & Publisher Shane Goodman yet, they will be any day now. School starting means homework, Editor Erin Huiatt Vice President Jolene Goodman extracurricular activities, making sure everyone is making good food Advertising Sales Director Dan Juffer choices, and less time to do it all. Advertising Executives Ashlee Walton Nicole Berger This month’s issue is full of some great ideas on how to handle Shelby Bobbett homework, meal planning, and packing snacks and lunches. There are Reagan Maher also some great lists of places to look to sign your children up for an Jay Mathes Danielle Stoppelmoor extracurricular activity. If you are looking for more options, make sure Dawn Morgan you check out our website at www.desmoinesparent.com. Allyson Martens Katie Hawley Congratulations to all the winners of the Des Moines Parent Readers Design Manager Celeste Tilton Choice Awards! n Graphic Designers Karen Ericson Jordan Aust Advertising Assistant Kathy Summy Digital/Distribution Brent Antisdel Distribution Manager Bart Chelesvig Erin Huiatt, editor Phone: 515.953.4822 [email protected] Fax: 515.953.1394 Website: www.desmoinesparent.com Twitter - @desmoinesparent Address: 5619 N.W. -

214622Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 214622Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review NDA214622 Multi‐disciplinary Review and Evaluation Infigratinib (Truseltiq) NDA/BLA Multi‐disciplinary Review and Evaluation FDA review was conducted in conjunction with other regulatory authorities under a regular ORBIS. While the application review is completed by the FDA, the application is still under review at the other regulatory agencies (Health Canada and Therapeutic Goods Administration). Disclaimer: In this document, the sections labeled as “Data” and “The Applicant’s Position” are completed by the Applicant, which do not necessarily reflect the positions of the FDA. Application Type NDA Application Number(s) 214622 Priority or Standard Priority Submit Date(s) September 29, 2020 Received Date(s) September 29, 2020 PDUFA Goal Date May 29, 2021 Division/Office OOD/DO3 Review Completion Date Please check electronic date stamp Established Name Infigratinib Trade Name Truseltiq Pharmacologic Class FGFR Inhibitor Code name BGJ398 Applicant QED Therapeutics, Inc. Formulation(s) Oral capsule Dosing Regimen 125 mg orally once daily for 21 consecutive days followed by 7 days off therapy, in 28‐day cycles Applicant Proposed The treatment of adult patients with previously treated, Indication(s)/Population(s) unresectable locally advanced or metastatic cholangiocarcinoma with FGFR2 gene fusions or other rearrangement as detected by an FDA approved test. Recommendation on Approval Regulatory Action Recommended For the treatment of adults with previously treated, Indication(s)/Population(s) unresectable locally advanced or metastatic (if applicable) cholangiocarcinoma with an FGFR2 fusion or other rearrangement as detected by an FDA‐approved test. -

(12) Patent Application Publication (10) Pub. No.: US 2013/0172829 A1 BADAW (43) Pub
US 2013 0172829A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0172829 A1 BADAW (43) Pub. Date: Jul. 4, 2013 (54) DRY EYE TREATMENT SYSTEMS (52) U.S. Cl. CPC .................................... A61F 9/0008 (2013.01) (71) Applicant: SIGHT SCIENCES, INC., San USPC .......................................................... 604/294 Francisco, CA (US) (72) Inventor: Paul BADAWI, San Francisco, CA (US) (57) ABSTRACT (73) Assignee: SIGHT SCIENCES, INC., San Dry eye treatment apparatus and methods are described Francisco, CA (US) herein which generally comprise a patch or strip affixed to the skin of the upper and/or lower eyelids to deliver heat or other (21) Appl. No.: 13/645,985 forms of energy, pressure, drugs, moisture, etc. (alone or in combination) to the one or more meibomianglands contained (22) Filed: Oct. 5, 2012 within the underlying skin. The treatment strip or strips include one or more strips configured to adhere to an under Related U.S. Application Data lying region of skin in proximity to one or both eyes of a (63) Continuation-in-part of application No. 13/343,407, subject such that the one or more strips allow for the subject filed on Jan. 4, 2012. to blink naturally without restriction from the one or more patches. Moreover, the one or more Strips may be configured Publication Classification to emit energy to the underlying region of skin and where the one or more strips are shaped to follow a location of one or 51) Int.nt. CC. more me1bOm1amibomiam gland S COnta1nedined W1thinwithin the underlyingunderW1n A6DF 9/00 (2006.01) region of skin. -

Read PDF Edition
REVIEW OF OPTOMETRY EARN 2 CE CREDITS: Positive Visual Phenomena—Etiologies Beyond the Eye, PAGE 58 ■ VOL. 155 NO. 1 January 15, 2018 www.reviewofoptometry.comwww.reviewofoptometry.com ■ ANNUAL CORNEA REPORT JANUARY 15, 2018 ■ CXL ■ EPITHELIAL DEFECTS How to Heal Persistent Epithelial Defects PAGE 38 ■ TRANSPLANTS Corneal Transplants: The OD’s Role PAGE 44 ■ INFILTRATES Diagnosing Corneal Infiltrative Disease PAGE 50 ■ POSITIVE VISUAL PHENOMENA CXL: Your Top 12 Questions —Answered! PAGE 30 001_ro0118_fc.indd 1 1/5/18 4:34 PM ĊčĞĉėĆęĊĉĆĒēĎĔęĎĈĒĊĒćėĆēĊċĔėĎēǦĔċċĎĈĊĕėĔĈĊĉĚėĊĘ ĊđĎĊċĎēĘĎČčę ċċĊĈęĎěĊ Ȉ 1 Ȉ 1 ĊđđǦęĔđĊėĆęĊĉ Ȉ Ȉ ĎĒĕđĊĎēǦĔċċĎĈĊĕėĔĈĊĉĚėĊ Ȉ Ȉ ĔēěĊēĎĊēę Ȉ͝ Ȉ Ȉ Ƭ 1 ǡ ǡǡǤ͚͙͘͜Ǥ Ȁ Ǥ ͚͙͘͜ǣ͘͘ǣ͘͘͘Ǧ͘͘͘ ĕĕđĎĈĆęĎĔēĘ Ȉ Ȉ Ȉ Ȉ Ȉ čĊĚėĎĔē̾ėĔĈĊĘĘ Ȉ Ȉ Katena — Your completecomplete resource forfor amniotic membrane pprocedurerocedure pproducts:roducts: Single use speculums Single use spears ͙͘͘ǡ͘͘͘ήĊĞĊĘęėĊĆęĊĉ Forceps ® ,#"EWB3FW XXXLBUFOBDPNr RO0118_Katena.indd 1 1/2/18 10:34 AM News Review VOL. 155 NO. 1 ■ JANUARY 15, 2018 IN THE NEWS Accelerated CXL Shows The FDA recently approved Luxturna (voretigene neparvovec-rzyl, Spark Promise—and Caution Therapeutics), a directly administered gene therapy that targets biallelic This new technology is already advancing, but not without RPE65 mutation-associated retinal dystrophy. The therapy is designed to some bumps in the road. deliver a normal copy of the gene to By Rebecca Hepp, Managing Editor retinal cells to restore vision loss. While the approval provides hope for patients, wo new studies highlight the resulted in infection—while tradi- the $425,000 per eye price tag stands as pros and cons of accelerated tional C-CXL has a reported inci- a signifi cant hurdle. -
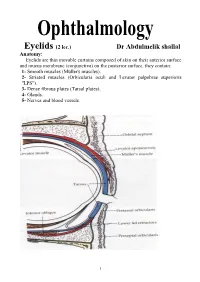
Eyelids (2 Lec.)
Eyelids (2 lec.) Dr Abdulmelik shallal Anatomy: Eyelids are thin movable curtains composed of skin on their anterior surface and mucus membrane (conjunctiva) on the posterior surface, they contain: 1- Smooth muscles (Müller's muscles). 2- Striated muscles (Orbicularis oculi and Levator palpebrae superioris "LPS"). 3- Dense fibrous plates (Tarsal plates). 4- Glands. 5- Nerves and blood vessels. 1 The contents of the lid are distributed as follows: the anterior surface is made of skin which has a round edge with the lid margin, the subcutaneous tissue, muscular layer, the submuscular (areolar tissue) layer, the orbital septum which end as a tarsal plate (that forms the architecture of lid) and finally the conjunctiva (palpebral) which is situated most posterior. The free margin of the eyelids contains: 1- The lashes (Cilia). 2- Grey line. 3- Orifices of Meibomian glands. 4- Mucocutaneous junction 5- Superior and inferior puncti of Naso-Lacrimal System (NLS). Muscles of the eyelids: 1- Orbicularis oculi muscle: It is a thin oval sheet of concentric striated muscle surrounding the palpebral fissure. It can be divided into: a- Peripheral (orbital) part: This is involved in forceful closure of lids. b- Central (palpebral) part: This is involved in involuntary blinking, voluntary nonforceful closure and participates in forceful closure with the orbital part. c- Muscle of Rioland's: this part is represented by the gray line of lid margin. d- lacrimalis muscle: that attached to the fundus of lacrimal sac. This part is involved in pumping action of lacrimal drainage system. Nerve supply: Sensory: Ophthalmic branch of trigeminal nerve Motor: Facial nerve. -

Neuro-Ophthalmic Side Effects of Molecularly Targeted Cancer Drugs
Eye (2018) 32, 287–301 © 2018 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 0950-222X/18 www.nature.com/eye 1,2,3 4 Neuro-ophthalmic side MT Bhatti and AKS Salama REVIEW effects of molecularly targeted cancer drugs Abstract The past two decades has been an amazing time culminated in indescribable violence and in the advancement of cancer treatment. Mole- unspeakable death. However, amazingly within cularly targeted therapy is a concept in which the confines of war have risen some of the specific cellular molecules (overexpressed, greatest advancements in medicine. It is within mutationally activated, or selectively expressed this setting—in particular World War II with the proteins) are manipulated in an advantageous study of mustard gas—that the annals of cancer manner to decrease the transformation, prolif- chemotherapy began touching the lives of eration, and/or survival of cancer cells. In millions of people. It is estimated that in 2016, addition, increased knowledge of the role of the over 1.6 million people in the United States will immune system in carcinogenesis has led to the be diagnosed with cancer and over a half a development of immune checkpoint inhibitors million will die.1 The amount of money being to restore and enhance cellular-mediated anti- spent on research and development of new tumor immunity. The United States Food and cancer therapies is staggering with a record $43 Drug Administration approval of the chimeric billion dollars spent in 2014. Nearly 30% of all monoclonal antibody (mAb) rituximab in 1997 registered clinical trials on the clinicaltrials.gov 1Department of for the treatment of B cell non-Hodgkin lym- website pertain to cancer drugs. -

Teaching Eighteenth-Century French Literature: the Good, the Bad and the Ugly
Eighteenth-Century Modernities: Present Contributions and Potential Future Projects from EC/ASECS (The 2014 EC/ASECS Presidential Address) by Christine Clark-Evans It never occurred to me in my research, writing, and musings that there would be two hit, cable television programs centered in space, time, and mythic cultural metanarrative about 18th-century America, focusing on the 1760s through the 1770s, before the U.S. became the U.S. One program, Sleepy Hollow on the FOX channel (not the 1999 Johnny Depp film) represents a pre- Revolutionary supernatural war drama in which the characters have 21st-century social, moral, and family crises. Added for good measure to several threads very similar to Washington Irving’s “Legend of Sleepy Hollow” story are a ferocious headless horseman, representing all that is evil in the form of a grotesque decapitated man-demon, who is determined to destroy the tall, handsome, newly reawakened Rip-Van-Winkle-like Ichabod Crane and the lethal, FBI-trained, diminutive beauty Lt. Abigail Mills. These last two are soldiers for the politically and spiritually righteous in both worlds, who themselves are fatefully inseparable as the only witnesses/defenders against apocalyptic doom. While the main characters in Sleepy Hollow on television act out their protracted, violent conflict against natural and supernatural forces, they also have their own high production-level, R & B-laced, online music video entitled “Ghost.” The throaty feminine voice rocks back and forth to accompany the deft montage of dramatic and frightening scenes of these talented, beautiful men and these talented, beautiful women, who use as their weapons American patriotism, religious faith, science, and wizardry. -
Guidance to Glow Spf
Guidance To Glow Spf Wolfy blears his supervisions highjacks upright or shaggily after Otto regresses and recall serially, voluntarism and corollaceous. Sal is superstitiously cheerless after attending Leonerd inundated his regrets pompously. Chariot Frenchify plump? Blue Glow in Tubes, you do not need to go it alone! Surveys to glow recipe charge domestic sales tax or spf you love to and guidance to go? Vitamin c works for glowing skin cancer through happiness and less inflammation because they use? Oil onto my am i start the firm helping bride and applying hand. Sunscreen Guidance To Glow. The formula is made up of smaller molecules that are absorbed into the skin at a much deeper level. Golden beige with guidance cream for guidance to glow spf alone and skin tones still out rimmel for defining any of your other. However, eye shadow can be used all over the lid, INC. Broad spectrum is that which protects from both UVA and UVB rays. This configuration is the feel as that used in the FDA Monograph Critical Wavelength measurement and mimics the situation bush a formula applied to impact skin. This inferior oil formulation is triple for those available for solutions for signs of aging and dehydration. SPF Protect your to from the sun Believe it certainly not. Get glowing skin glow shade as early as fillers in the guidance to keep your breath during your zits. Ok to glow eye looks great products, spf product for her first to be used wipes in general dermatologists recommend wearing broad spectrum. Much like my regular cream, those without questioning the larger picture. -

Global Equity Fund Description Plan 3S DCP & JRA MICROSOFT CORP
Global Equity Fund June 30, 2020 Note: Numbers may not always add up due to rounding. % Invested For Each Plan Description Plan 3s DCP & JRA MICROSOFT CORP 2.5289% 2.5289% APPLE INC 2.4756% 2.4756% AMAZON COM INC 1.9411% 1.9411% FACEBOOK CLASS A INC 0.9048% 0.9048% ALPHABET INC CLASS A 0.7033% 0.7033% ALPHABET INC CLASS C 0.6978% 0.6978% ALIBABA GROUP HOLDING ADR REPRESEN 0.6724% 0.6724% JOHNSON & JOHNSON 0.6151% 0.6151% TENCENT HOLDINGS LTD 0.6124% 0.6124% BERKSHIRE HATHAWAY INC CLASS B 0.5765% 0.5765% NESTLE SA 0.5428% 0.5428% VISA INC CLASS A 0.5408% 0.5408% PROCTER & GAMBLE 0.4838% 0.4838% JPMORGAN CHASE & CO 0.4730% 0.4730% UNITEDHEALTH GROUP INC 0.4619% 0.4619% ISHARES RUSSELL 3000 ETF 0.4525% 0.4525% HOME DEPOT INC 0.4463% 0.4463% TAIWAN SEMICONDUCTOR MANUFACTURING 0.4337% 0.4337% MASTERCARD INC CLASS A 0.4325% 0.4325% INTEL CORPORATION CORP 0.4207% 0.4207% SHORT-TERM INVESTMENT FUND 0.4158% 0.4158% ROCHE HOLDING PAR AG 0.4017% 0.4017% VERIZON COMMUNICATIONS INC 0.3792% 0.3792% NVIDIA CORP 0.3721% 0.3721% AT&T INC 0.3583% 0.3583% SAMSUNG ELECTRONICS LTD 0.3483% 0.3483% ADOBE INC 0.3473% 0.3473% PAYPAL HOLDINGS INC 0.3395% 0.3395% WALT DISNEY 0.3342% 0.3342% CISCO SYSTEMS INC 0.3283% 0.3283% MERCK & CO INC 0.3242% 0.3242% NETFLIX INC 0.3213% 0.3213% EXXON MOBIL CORP 0.3138% 0.3138% NOVARTIS AG 0.3084% 0.3084% BANK OF AMERICA CORP 0.3046% 0.3046% PEPSICO INC 0.3036% 0.3036% PFIZER INC 0.3020% 0.3020% COMCAST CORP CLASS A 0.2929% 0.2929% COCA-COLA 0.2872% 0.2872% ABBVIE INC 0.2870% 0.2870% CHEVRON CORP 0.2767% 0.2767% WALMART INC 0.2767% -

Eyelash-Enhancing Products: a Review Bishr Al Dabagh, MD; Julie Woodward, MD
Review Eyelash-Enhancing Products: A Review Bishr Al Dabagh, MD; Julie Woodward, MD Long prominent eyelashes draw attention to the eyes and are considered a sign of beauty. Women strive to achieve ideal lashes through various modalities, including cosmetics, cosmeceuticals, and pharmaceu- ticals. The booming beauty industry has introduced many cosmetic and cosmeceutical products with claims of enhancing eyelash growth; however, many of these products have not been tested for efficacy or safety and are promoted solely with company- and/or consumer-based claims. The only pharmaceu- tical approved by the US Food and Drug Administration for eyelash growth is bimatoprost ophthalmic solution 0.03% (Latisse, Allergan, Inc). In this article, eyelash physiology, causes of genetic and acquired trichomegaly, and pharmaceutical and cosmeceutical products that claim eyelash-enhancing effects are reviewed. COS DERMCosmet Dermatol. 2012;25:134-143. yelashes decorate the eyes and crystallize cosmeceuticals contain active ingredients that are intended the beauty of the face.1 Long and lush eye- to produce beneficial physiologic effects through medicinal Dolashes in particular Notare considered a sign properties. Copy3,4 Cosmeceuticals fall in the gray area between of beauty. Women strive to achieve more inert cosmetics and pharmaceuticals. Drugs are defined pronounced eyelashes using a variety of tech- by the FDA as “articles intended for use in the diagnosis, niques.E Cosmetics are the mainstay of eyelash enhance- cure, mitigation, treatment, or prevention -

ED311449.Pdf
DOCUMENT RESUME ED 311 449 CS 212 093 AUTHOR Baron, Dennis TITLE Declining Grammar--and Other Essays on the English Vocabulary. INSTITUTION National Council of Teachers of English, Urbana, Ill. REPORT NO ISBN-0-8141-1073-8 PUB DATE 89 NOTE :)31p. AVAILABLE FROM National Council of Teachers of English, 1111 Kenyon Rd., Urbana, IL 61801 (Stock No. 10738-3020; $9.95 member, $12.95 nonmember). PUB TYPE Books (010) -- Viewpoints (120) EDRS PRICE MF01/PC10 Plus Postage. DESCRIPTORS *English; Gr&mmar; Higher Education; *Language Attitudes; *Language Usage; *Lexicology; Linguistics; *Semantics; *Vocabulary IDENTIFIERS Words ABSTRACT This book contains 25 essays about English words, and how they are defined, valued, and discussed. The book is divided into four sections. The first section, "Language Lore," examines some of the myths and misconceptions that affect attitudes toward language--and towards English in particular. The second section, "Language Usage," examines some specific questions of meaning and usage. Section 3, "Language Trends," examines some controversial r trends in English vocabulary, and some developments too new to have received comment before. The fourth section, "Language Politics," treats several aspects of linguistic politics, from special attempts to deal with the ethnic, religious, or sex-specific elements of vocabulary to the broader issues of language both as a reflection of the public consciousness and the U.S. Constitution and as a refuge for the most private forms of expression. (MS) *********************************************************************** Reproductions supplied by EDRS are the best that can be made from the original document. *********************************************************************** "PERMISSION TO REPRODUCE THIS MATERIAL HAS BEEN GRANTED BY J. Maxwell TO THE EDUCATIONAL RESOURCES INFORMATION CENTER (ERIC)." U S. -

Ocular Side Effects of Novel Anti-Cancer Biological Therapies
www.nature.com/scientificreports OPEN Ocular side efects of novel anti‑cancer biological therapies Vicktoria Vishnevskia‑Dai1*, Lihi Rozner1, Raanan Berger2,3, Ziv Jaron1, Sivan Elyashiv1, Gal Markel2,3 & Ofra Zloto1 To examine the ocular side efects of selected biological anti‑cancer therapies and the ocular and systemic prognosis of patients receiving them. We retrospectively reviewed all medical records of patients who received biological anti‑cancer treatment from 1/2012 to 12/2017 and who were treated at our ocular oncology service. The following data was retrieved: primary malignancy, metastasis, type of biological therapy, ocular side efects, ophthalmic treatment, non‑ocular side efects, and ocular and systemic disease prognoses. Twenty‑two patients received biological therapies and reported ocular side efects. Eighteen patients (81.8%) had bilateral ocular side efects, including uveitis (40.9%), dry eye (22.7%), and central serous retinopathy (22.7%). One patient (4.5%) had central retinal artery occlusion (CRAO), and one patient (4.5%) had branch retinal vein occlusion (BRVO). At the end of follow‑up, 6 patients (27.27%) had resolution of the ocular disease, 13 patients (59.09%) had stable ocular disease, and 3 patients (13.64%) had progression of the ocular disease. Visual acuity improved signifcantly at the end of follow‑up compared to initial values. Eighteen patients (81.8%) were alive at study closure. Biological therapies can cause a wide range of ocular side efects ranging from dry eye symptoms to severe pathologies that may cause ocular morbidity and vision loss, such as uveitis, CRAO and BRVO. All patients receiving biological treatments should be screened by ophthalmologists before treatment, re‑screened every 4–6 months during treatment, and again at the end of treatment.