A Unification Theory for Dry Eye and Blepharitis Open Access to Scientific and Medical Research DOI
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2013/0172829 A1 BADAW (43) Pub
US 2013 0172829A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0172829 A1 BADAW (43) Pub. Date: Jul. 4, 2013 (54) DRY EYE TREATMENT SYSTEMS (52) U.S. Cl. CPC .................................... A61F 9/0008 (2013.01) (71) Applicant: SIGHT SCIENCES, INC., San USPC .......................................................... 604/294 Francisco, CA (US) (72) Inventor: Paul BADAWI, San Francisco, CA (US) (57) ABSTRACT (73) Assignee: SIGHT SCIENCES, INC., San Dry eye treatment apparatus and methods are described Francisco, CA (US) herein which generally comprise a patch or strip affixed to the skin of the upper and/or lower eyelids to deliver heat or other (21) Appl. No.: 13/645,985 forms of energy, pressure, drugs, moisture, etc. (alone or in combination) to the one or more meibomianglands contained (22) Filed: Oct. 5, 2012 within the underlying skin. The treatment strip or strips include one or more strips configured to adhere to an under Related U.S. Application Data lying region of skin in proximity to one or both eyes of a (63) Continuation-in-part of application No. 13/343,407, subject such that the one or more strips allow for the subject filed on Jan. 4, 2012. to blink naturally without restriction from the one or more patches. Moreover, the one or more Strips may be configured Publication Classification to emit energy to the underlying region of skin and where the one or more strips are shaped to follow a location of one or 51) Int.nt. CC. more me1bOm1amibomiam gland S COnta1nedined W1thinwithin the underlyingunderW1n A6DF 9/00 (2006.01) region of skin. -
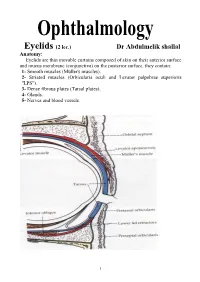
Eyelids (2 Lec.)
Eyelids (2 lec.) Dr Abdulmelik shallal Anatomy: Eyelids are thin movable curtains composed of skin on their anterior surface and mucus membrane (conjunctiva) on the posterior surface, they contain: 1- Smooth muscles (Müller's muscles). 2- Striated muscles (Orbicularis oculi and Levator palpebrae superioris "LPS"). 3- Dense fibrous plates (Tarsal plates). 4- Glands. 5- Nerves and blood vessels. 1 The contents of the lid are distributed as follows: the anterior surface is made of skin which has a round edge with the lid margin, the subcutaneous tissue, muscular layer, the submuscular (areolar tissue) layer, the orbital septum which end as a tarsal plate (that forms the architecture of lid) and finally the conjunctiva (palpebral) which is situated most posterior. The free margin of the eyelids contains: 1- The lashes (Cilia). 2- Grey line. 3- Orifices of Meibomian glands. 4- Mucocutaneous junction 5- Superior and inferior puncti of Naso-Lacrimal System (NLS). Muscles of the eyelids: 1- Orbicularis oculi muscle: It is a thin oval sheet of concentric striated muscle surrounding the palpebral fissure. It can be divided into: a- Peripheral (orbital) part: This is involved in forceful closure of lids. b- Central (palpebral) part: This is involved in involuntary blinking, voluntary nonforceful closure and participates in forceful closure with the orbital part. c- Muscle of Rioland's: this part is represented by the gray line of lid margin. d- lacrimalis muscle: that attached to the fundus of lacrimal sac. This part is involved in pumping action of lacrimal drainage system. Nerve supply: Sensory: Ophthalmic branch of trigeminal nerve Motor: Facial nerve. -

Procedural Article
Procedural Article The occasional eyelid lesion Mitchell Crozier, INTRODUCTION ANATOMY/ETIOLOGY BHK1, Sarah M. Giles, BSc, Physicians in the primary and External hordeola originate from an MD2 urgent care settings frequently acute staphylococcal infection of the 1School of Medicine, Faculty encounter patients presenting with sebaceous glands (Glands of Zeiss) or of Medicine, acute inflammatory eyelid nodules modified apocrine glands (Glands of University of Ottawa, and eyelid swelling. The external Moll) found along the margin of the Ottawa, ON, Canada, hordeolum, which is a painful infection upper and lower eyelid.3,4 Together, 2Department of Family Medicine, Faculty of involving the eyelid and referred the Glands of Zeiss and Moll produce Medicine, University of to as a ‘stye’ in clinical practice, is secretions with antibacterial and Ottawa, Ottawa, ON, one of the most common eye/eyelid immune defence properties.1,4,8 The Canada conditions reported by the general Glands of Zeis secrete into a duct at 1‑3 Correspondence to: population. There are no known the base of the eyelash hair follicle, Sarah M. Giles, age, sex or demographic differences while the Glands of Moll secrete [email protected] in the prevalence of external hordeola directly to the eyelid surface next to but patients with chronic conditions the base of the eyelashes and anterior This article has been peer 8 reviewed such as diabetes, dyslipidaemia and to the meibomian glands. When the seborrheic dermatitis may be at an glands become blocked, or if stasis increased risk.4,5 occurs, bacterial proliferation and Patients with an external infection can occur. As the infection hordeolum present with an acute‑onset results in a localised inflammatory red, painful and swollen abscess along response, a purulent and palpable the margin of the eyelid. -

Biomarkers in Sebaceous Gland Carcinomas
3/24/2017 Biomarkers in Sebaceous Gland Carcinomas Sander R. Dubovy, MD Professor of Ophthalmology and Pathology Victor T. Curtin Chair in Ophthalmology Florida Lions Ocular Pathology Laboratory Bascom Palmer Eye Institute University of Miami Miller School of Medicine Biomarkers in Sebaceous Gland Carcinomas Disclosure of Relevant Disclosure of Relevant Financial Relationships Financial Relationships USCAP requires that all planners (Education Committee) in a position to Dr. Sander R. Dubovy declares he has no conflict(s) of interest influence or control the content of CME disclose any relevant financial to disclose. relationship WITH COMMERCIAL INTERESTS which they or their spouse/partner have, or have had, within the past 12 months, which relates to the content of this educational activity and creates a conflict of interest. Biomarkers in Sebaceous Gland Carcinomas Outline Introduction • Sebaceous carcinoma (SC) is a malignant neoplasm that arises from • Introduction to sebaceous cell carcinoma the sebaceous glands, most commonly in the periocular areas. • Incidence, demographics, risk factors • Clinical manifestations are often mistaken for benign conditions and • Ocular origins thus proper diagnosis and management is delayed. • Gross pathology • Metastases to regional lymph nodes and other sites are common. • Microscopic pathology • Immunohistochemistry • Management • Cases Biomarkers in Sebaceous Gland Carcinomas Biomarkers in Sebaceous Gland Carcinomas 1 3/24/2017 Introduction Sebaceous Gland • Pathologists should be aware of the -

Ophthalmology
LECTURE NOTES For Health Science Students Ophthalmology Dereje Negussie, Yared Assefa, Atotibebu Kassa, Azanaw Melese University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2004 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2004 by Dereje Negussie, Yared Assefa, Atotibebu Kassa, Azanaw Melese All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE This lecture note will serve as a practical guideline for the hard-pressed mid-level health workers. We hope that it will be a good introduction to eye diseases for health science students working in Ethiopia. -

Differential Incidence of Eyelid Margin Cysts in ASU Outpatients
The Egyptian Journal of Hospital Medicine (July 2018) Vol. 72 (6), Page 4761-4764 Differential Incidence of Eyelid Margin Cysts in ASU Outpatients Mayar Mohammad Salaheldin Faheem, Sherif Elwan, Azza Mohamed Ahmed Said, Ossama Tarek Nada Department of Ophthalmology, Faculty of Medicine, Ain Shams University Corresponding author: Mayar Mohammad Salaheldin Faheem, E-Mail: [email protected], Mobile: +2 01097316366 ABSTRACT Background: Wide varieties of lesions affecting the eyelid are encountered within routine Ophthalmology practice. These lesions are numerous due to the unique anatomical features of the eyelid as the whole skin structures, appendages, muscle, modified glands, and conjunctival mucous membrane are represented in the eyelid. The eyelid comprises different types of glands that clinically correlate with the development of eyelid cysts; Sebaceous glands (Meibomian glands and glands of Zeis), aporcine glands (glands of Moll) and eccrine sweat glands. Objectives: To report the incidence of the different etiologies of eyelid margin cysts in Ain Shams University (ASU) Hospital, General Ophthalmology Outpatients Clinic. Patients and Methods: It included all patients who presented to the General Ophthalmology Outpatient Clinic, 6 days per week for one month (November 2017) complaining of eyelid margin lesions, found cystic on slit lamp examination, of all ages. Results: The total number of patients recorded was 45 patients presenting with eyelid margin cysts, out of 1920 patients visiting the General Ophthalmology Outpatient Clinic during the month of November 2017 with an incidence of 2.34%. Seven types of cysts were found namely; External Hordeolum (8.9%), Internal Hordeolum (15.6%), Chalazion (28.9%), Sebaceous Cyst (11.1%), Cyst of Zeis Gland (13.3%), Apocrine Hidrocystoma (11.1%) and Eccrine Hidrocystoma (11.1%). -

Benign Eye Lid Lesions Arnab Biswas1
Review Article Benign eye lid lesions Arnab Biswas1 (Excerpts from Eyelid Tumor Clinical Evaluation and Reconstruction Techniques Authors: Biswas, Springer; 2014 edition) ny Eyelid mass or ulcer could be a benign or a Seboric Keratosis malignant lesion. An accurate diagnosis can be A Introduction: Seborrheic keratosis( Basal cell reached based on history and clinical examination. If in papilloma,seborrheic warts) are common benign lesions doubt surgical biopsy followed by histo pathological on the face and abdomen. It can also present on the lids evaluation can clinch the diagnosis. In this review article of aging individuals. we look at some of the more common eyelid lesions that an ophthalmologist may encounter in a general practice. Clinical Features: They are well circumscribed, waxy, friable and appear stuck on to the skin. Some lesions are covered BENIGN TUMORS OF EPIDERMIS by an adherent greasy-appearing scale and are raised Squamous Papilloma above the surface of the skin. They can feel soft and greasy. Introduction: It is one of the most common benign eye lid The shape is round to oval, and multiple lesions may be lesion. It is not a specific clino-pathological entity. aligned in the direction of skin folds.The lesion is very superficial and may be pigmented from slight discolouration to deep brown in colour. Prognosis: They are usually assymptomatic, but can sometimes cause pruritis and irritation. Treatment Options: Treatment involves surgical excision or laser ablation Inverted Folicular Keratosis Introduction: It is a benign cutaneous lesion almost similar in character to seborrheic keratosis. The term Inverted A large pailomatous growth from lateral aspect of lower lid follicular karatosis is a misnomer, as it was thought that Age of Presentation: It is a group of condition that usually presents in middle or elderly age. -

Sensitive Cilia – Eyelashes in Health and Disease
FEATURE Sensitive cilia – eyelashes in health and disease BY RACHNA MURTHY AND JONATHAN ROOS In health our eyelashes protect the eyes, but in disease they can disfigure, impair quality of life and threaten vision. In this review the authors discuss aspects of lashes that are relevant to all professionals working near the eyes and how to keep you and your patients safe. ur eyelashes frame the eyes and are a key facial aesthetic: of the protein keratin also containing melanin and an outermost we have evolved to be preoccupied by the periocular area impermeable cuticle of several layers of scale-like cells. They when we meet someone. Eye tracking studies show that are coated with sebum secreted by a gland named after Dr Zeis Oour gaze is primarily focussed on the eyes and periocular (Figure 1). Like all hair, lashes transition through three phases in area when looking at one another [1]. When we look into someone’s their life but with a short anagen growth phase and long resting eyes we feel we can see their soul – but except for pupil size the eye telogen phase (Figure 2). This means that they reach no more than itself does not change – rather it is the surrounding tissues which around 10mm before growth stops. They then shed naturally after a speak to us. few months. One to four lashes are lost per day. Eyelashes tend to be The eyelashes contribute significantly to this aesthetic and the darkest hairs in the body and the last ones to undergo greying, communication; whole industries have been spawned for their usually late in life [11]. -
The Redred Eyeeye GPGP Updateupdate 20102010 -- Mrmr Vaughanvaughan Tannertanner
TheThe RedRed EyeEye GPGP UpdateUpdate 20102010 -- MrMr VaughanVaughan TannerTanner www.tanner-eyes.co.uk ReadingReading WindsorWindsor Royal Berkshire Hospital Prince Charles Eye Unit Dunedin Hospital Princess Margaret Hospital Lids Conjunctiva Duration ? Sclera Cornea Uveitis Is it painful ? Glaucoma Others Is vision decreased ? StaphylococcalStaphylococcal blepharitisblepharitis • Chronic irritation • Hyperaemia and telangiectasia • Worse in mornings of anterior lid margin • Scales around base of lashes (collarettes) • Scarring and hypertrophy ComplicationsComplications ofof staphylococcalstaphylococcal blepharitisblepharitis Trichiasis Recurrent styes Tear film Marginal instability keratitis TreatmentTreatment ofof ChronicChronic BlepharitisBlepharitis 1. Lid hygiene – clean debris from lashes at night with cotton bud 2.Chloramphenicol Ointment – to lid margins at night 3. Tear substitutes - for associated tear film instability Hypromellose, Optive, Celluvisc 4. Oral Lymecycline 408 mg OD one month – very useful in most cases The Red Eye - Mr Vaughan Tanner CONJUNCTIVALCONJUNCTIVAL INFECTIONSINFECTIONS 1. Bacterial • Simple bacterial conjunctivitis 2. Viral • Adenoviral keratoconjunctivitis • Molluscum contagiosum conjunctivitis • Herpes simplex conjunctivitis 3. Chlamydial • Adult chlamydial keratoconjunctivitis • Neonatal chlamydial conjunctivitis • Trachoma The Red Eye - Mr Vaughan Tanner SimpleSimple bacterialbacterial conjunctivitisconjunctivitis Crusted eyelids and conjunctival mucopurulent discharge injection - broad-spectrum -

The Final Front Tear
1/30/2021 1. Terminology related to dry eyes 2. Anatomy of the eye 3. Anatomy of the tear and impact on vision 4. Clinical evaluation of the ocular The Final Objectives surface 5. Causes of dry eyes Front Tear 6. Tear testing Lynn E. Lawrence, CMSgt(ret), USAF 7. Dry eye treatment MSOL, CPOT, ABOC, COA, OSC Anatomy • Dry Eye • Inflammatory Dry Eye • Aqueous Insufficiency • Evaporative Dry Eye • Sjogrens Disease • Keratitis Sicca • Osmolarity • Tear Break-Up Time Punctum What function does the punctum have? Terms Industry Issues Terms • AD Aqueous Deficiency • OSD - Ocular Surface • DED – Dry Eye Disease Disease • If left undiagnosed, this can cause • DES – Dry Eye Syndrome • OSDI - Ocular Surface • Patients are leaving the office Disease Index complications with eye surgery • DEWS – Dry Eye Work undiagnosed in too many cases • POTF – Pre-Ocular Tear Shop Film • Systemic diseases can exacerbate the • DTS – Dysfunctional Tear • Patients are not volunteering the System • SPEED – Standard issue Patient Evaluation of Eye necessary information • Lipid Insufficiency Dryness • MDG – Meibomian • Medications can cause a significant • Expression • Gland Dysfunction Staff members are not asking the decline in the condition • Homeostasis of the tear necessary questions • NLDO – Nasal Lacrimal film Duct Obstruction • Symptoms correlation • Too many products to choose from • Too much mis-information 1 1/30/2021 Anatomy and Physiology of the ocular adnexa • Eyelids • Corneal • Eyebrows sensitivity • Eyelashes (permanent make- up) • Lacrimal gland innovation • Accessory glands • Lacrimal Apparatus th What is the opening between 5 Cranial Nerve - the upper and lower lid called? Trigeminal Lacrimal Apparatus • Sometimes a person cannot produce natural tears they might need punctal plugs to prevent the tears from draining off the eye. -

Cataract Confusion Clarified Ken Abrams, DVM, DACVO Eye Care for Animals Warwick, RI
Cataract Confusion Clarified Ken Abrams, DVM, DACVO Eye Care for Animals Warwick, RI Anatomy The canine lens is suspended in the eye by zonular ligaments which arise from the ciliary epithelium and attach to the lens at the equator. The lens capsule is an elastic basement membrane which encloses the underlying anterior epithelium and lens fibers. The size of the lens is approximately 7mm in the anterior/posterior aspect and 10mm as the equatorial diameter. The anterior lens capsule is much thicker (50-70um) than the posterior capsule (2-4 um) and is an important factor when performing cataract extraction. Lens fibers run parallel to each other, running from anterior to posterior. However, the fibers don’t quite reach the anterior and posterior poles or cover the entire circumference and thereby form the upright, anterior ‘Y’ suture and inverted, posterior ‘Y’ suture. The lens is nourished in the embryo by the hyaloid artery arising from the posterior segment and later in life, by the aqueous humor and vitreous. Mittendorf’s dot represents the insertion of the hyaloid artery on to the posterior lens capsule. Congenital lesions of the lens Most congenital lesions of the canine lens involve a failure of atrophy of fetal vascular structures. The problem can be as minor as the presence of Mittendorf’s dot, representing the insertion of the hyaloid artery on the posterior lens capsule. An avascular tail leading from Mittendorf’s dot can be seen in the vitreous in many patients. Persistent hyperplastic primary vitreous (PHPV) is the most severe form of failure of atrophy of fetal lenticular vessels and is an inherited disease in Dobermans and Bull Terriers. -
TNA, Chapter III
TERMINOLOGIA NEUROANATOMICA International Neuroanatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TNA, Chapter III Contents Caput III: Organa sensuum Chapter 3: Sense organs Organum olfactorium Olfactory organ Organum visuale (Oculus) Visual organ (Eye) Organum vestibulocochleare (Auris) Vestibulocochlear organ (Ear) Organum gustatorium Gustatory organ Bibliographic Reference Citation: FIPAT. Terminologia Neuroanatomica. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Neuroanatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput III: ORGANA SENSUUM Chapter 3: SENSE ORGANS Latin term Latin synonym UK English US English English synonym Other 3579 Organa