1.0 Anatomy and Physiology of Salmonids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

O2 Secretion in the Eye and Swimbladder of Fishes
1641 The Journal of Experimental Biology 209, 1641-1652 Published by The Company of Biologists 2007 doi:10.1242/jeb.003319 Historical reconstructions of evolving physiological complexity: O2 secretion in the eye and swimbladder of fishes Michael Berenbrink School of Biological Sciences, The University of Liverpool, Biosciences Building, Crown Street, Liverpool, L69 7ZB, UK e-mail: [email protected] Accepted 12 March 2007 Summary The ability of some fishes to inflate their compressible value of haemoglobin. These changes predisposed teleost swimbladder with almost pure oxygen to maintain neutral fishes for the later evolution of swimbladder oxygen buoyancy, even against the high hydrostatic pressure secretion, which occurred at least four times independently several thousand metres below the water surface, has and can be associated with increased auditory sensitivity fascinated physiologists for more than 200·years. This and invasion of the deep sea in some groups. It is proposed review shows how evolutionary reconstruction of the that the increasing availability of molecular phylogenetic components of such a complex physiological system on a trees for evolutionary reconstructions may be as important phylogenetic tree can generate new and important insights for understanding physiological diversity in the post- into the origin of complex phenotypes that are difficult to genomic era as the increase of genomic sequence obtain with a purely mechanistic approach alone. Thus, it information in single model species. is shown that oxygen secretion first evolved in the eyes of fishes, presumably for improved oxygen supply to an Glossary available online at avascular, metabolically active retina. Evolution of this http://jeb.biologists.org/cgi/content/full/210/9/1641/DC1 system was facilitated by prior changes in the pH dependence of oxygen-binding characteristics of Key words: oxygen secretion, Root effect, rete mirabile, choroid, haemoglobin (the Root effect) and in the specific buffer swimbladder, phylogenetic reconstruction. -

Respiratory Disorders of Fish
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Disorders of the Respiratory System in Pet and Ornamental Fish a, b Helen E. Roberts, DVM *, Stephen A. Smith, DVM, PhD KEYWORDS Pet fish Ornamental fish Branchitis Gill Wet mount cytology Hypoxia Respiratory disorders Pathology Living in an aquatic environment where oxygen is in less supply and harder to extract than in a terrestrial one, fish have developed a respiratory system that is much more efficient than terrestrial vertebrates. The gills of fish are a unique organ system and serve several functions including respiration, osmoregulation, excretion of nitroge- nous wastes, and acid-base regulation.1 The gills are the primary site of oxygen exchange in fish and are in intimate contact with the aquatic environment. In most cases, the separation between the water and the tissues of the fish is only a few cell layers thick. Gills are a common target for assault by infectious and noninfectious disease processes.2 Nonlethal diagnostic biopsy of the gills can identify pathologic changes, provide samples for bacterial culture/identification/sensitivity testing, aid in fungal element identification, provide samples for viral testing, and provide parasitic organisms for identification.3–6 This diagnostic test is so important that it should be included as part of every diagnostic workup performed on a fish. -
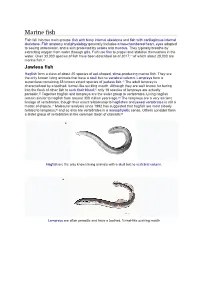
Marine Fish Fish Fall Into Two Main Groups: Fish with Bony Internal Skeletons and Fish with Cartilaginous Internal Skeletons
Marine fish Fish fall into two main groups: fish with bony internal skeletons and fish with cartilaginous internal skeletons. Fish anatomy and physiology generally includes a two-chambered heart, eyes adapted to seeing underwater, and a skin protected by scales and mucous. They typically breathe by extracting oxygen from water through gills. Fish use fins to propel and stabilise themselves in the water. Over 33,000 species of fish have been described as of 2017,[1] of which about 20,000 are marine fish.[2] Jawless fish Hagfish form a class of about 20 species of eel-shaped, slime-producing marine fish. They are the only known living animals that have a skull but no vertebral column. Lampreys form a superclass containing 38 known extant species of Jawless fish.[3] The adult lamprey is characterized by a toothed, funnel-like sucking mouth. Although they are well known for boring into the flesh of other fish to suck their blood,[4] only 18 species of lampreys are actually parasitic.[5] Together hagfish and lampreys are the sister group to vertebrates. Living hagfish remain similar to hagfish from around 300 million years ago.[6] The lampreys are a very ancient lineage of vertebrates, though their exact relationship to hagfishes and jawed vertebrates is still a matter of dispute.[7] Molecular analysis since 1992 has suggested that hagfish are most closely related to lampreys,[8] and so also are vertebrates in a monophyletic sense. Others consider them a sister group of vertebrates in the common taxon of craniata.[9] • Hagfish are the only known living animals with a skull but no vertebral column. -

Ventilation Systems • Cilliary Action • Muscle Pumps – Buccal Pumps – Body Wall • Body Positioning • Ram Ventilation • Negative Pressure Diaphragms
3/27/2017 Ventilation • Increase exchange rate by moving medium over respiratory surface • Water – ~800x heavier than air, potentially expensive ventilation – Oxygen per unit mass • Air: 1.3 g contains 210 ml O2 • Water: 1000 g contains 5-10 ml O2 • Air – diffusion can only occur once gasses dissolved, all respiratory surfaces must be wet – Respiratory flux Systems • Ventilation system - devote resources to anatomical structures or behaviors aimed at increasing ventilation and thus potential gas exchange • Circulatory system – devote resources to anatomical structures to more efficiently move gasses (and other things) around the body – More efficient control and delivery 1 3/27/2017 Types of Ventilation Systems • Cilliary action • Muscle pumps – Buccal pumps – Body wall • Body positioning • Ram ventilation • Negative pressure diaphragms Air/Water Interaction with Surface 2 3/27/2017 Types of respiratory structures • Skin (cutaneous) • Gills – protruding structure, increases surface area – Tuft – Filament – Lamellar • Trachea – internalized but not centralized • Lung – internalized and centralized structure Cutaneous exchange • Most animals get some oxygen this way • Requires thin, moist skin • Flow of deoxygenated body fluids near skin surface • Ontogenetic trends – most larval fish do not use gills • Some secondary loss of lungs (Plethodontidae) • Ventilation (Lake Titicaca frog) 3 3/27/2017 Trachea systems • Multiple independent tubes • Spriacles for ventilation • Branches supply tissues directly • Some connected to “gills” • Body movement for ventilation Fish Gills • Ventilation – One way flow – pumps: buccal, opercular, stomach, esophageal – ram ventilation • Ventilation to profusion ratio = volume pumped to volume in contact with gills – Higher value requires more gill surface area 4 3/27/2017 • Types of gills – Holobranch: lamellae on both sides of the fillament – Hemibranch: lamellae on one side – Pseudobranch: no true lamellae Gills 5 3/27/2017 Gill area • Greater in marine than freshwater • Greater for more metabolically active fish (e.g. -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

Explore a Fish Lesson Plan
Grade Level 4 - 12 EXPLORE A FISH Duration FISH DISSECTION 1 - 1 ½ hours Subject/Subject Area Focus Overview Science, language arts; Students will examine the external and internal anatomy of various fish Analysis, application, species. They will note similarities and differences. Students will then communication, use their observations to make inferences about the relationships among comparing similarities and them. differences, description, discussion, drawing, small Background information group work, using time This activity gives students first-hand experience exploring the and space, writing. adaptations which allow fish to function in their environment. Students look at both form and function of different systems to help understand Materials how specific adaptations assist organisms in adapting to their For each pair (or group) of environment. How do fish move through the water and keep their vertical students: position within the water? Students can make comparisons between their • Whole body fresh fish own anatomy and the anatomy of a fish. • Dissecting trays or thick pads of newspaper It is important for students to understand the purpose of this activity • Scissors or Scalpel is to study the internal and external anatomy of a fish. It requires (Most cuts can be concentration, listening skills and being able to follow directions. All made with a pair of students should be given the option of not participating in the activity classroom scissors) and be allowed an alternate activity. You may want to do a practice run on • Probe (A large partially your own. straightened paper clip works well) You can either do this activity as a teacher-led class discussion or break • Forceps (A nice tool but the students into cooperative learning groups and give each group: a fish, not necessary) “Explore a Fish” worksheet, dissection and anatomy sheet, newspaper • Paper towels and and something to cut the fish with. -

The Mouth and Gastrointestinal Tract of the African Lung Fish Protopterus Annectens (Owen 1839) in River Niger at Agenebode, Edo State Nigeria
Egyptian Journal of Aquatic Biology & Fisheries Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt. ISSN 1110 – 6131 Vol. 23(4): 181- 188 (2019) www.ejabf.journals.ekb.eg The mouth and gastrointestinal tract of the african lung fish Protopterus annectens (owen 1839) in River Niger at Agenebode, Edo State Nigeria Agbugui Marian Onwude1* and Oniye Sunnie Joshua2 1-Department of Biological Sciences, Edo University Iyamho, Edo State, Nigeria 2-Department of Zoology, Ahmadu Bello University, Zaria, Kaduna State, Nigeria *Corresponding Author: [email protected] ARTICLE INFO ABSTRACT Article History: The mouth of P. annectens is terminal, its jaws are modified to hold Received: May 24, 2019 incisors -scissor-like teeth that can hold and tear deep into the flesh of prey. Accepted: Sept. 28, 2019 The mouth opens up to 10% of its total body length which allows the fish to Online: Oct. 2019 seize its prey easily. The gastrointestinal (GIT) is straight, short and a _______________ composite comprising of many organs and tissues. The organs are wrapped in serosa. The morphology and histology of the GIT, (oesophagus to the cloaca) Keywords: reveals the adaptation to the food and feeding habit of fish. The numerous Protopterus annectens African lung fish mucosal folds in the intestine are a usual occurrence in carnivorous species. River Niger The ability of the intestine to distend and the presence of the numerous large Agenebode folds allow fishes to accommodate a large quantity of food items as digestion Gastrointestinal tract takes place and nutrients are being ingested. INTRODUCTION The West African Lung fish are an important source of food supply and protein for man. -

Fact Sheet: Fish Anatomy
Fact Sheet: Fish Anatomy WA Curriculum K-10 Science, SS Biology – ATAR, SS Biology – General, SS Integrated Science – ATAR, SS Integrated Science – General Region North Coast, Gascoyne Coast, West Coast, South Coast, Indian Ocean Territories Summary Fishes are a large and varied group of aquatic animals. Worldwide, there are over 32,000 described species, with over 4,400 in Australia (Australian Museum). They come in an amazing variety of shapes and sizes. All fishes have the following features in common: they live in water; have a backbone (vertebrate); and breathe using gills. Most fishes also: have scales; move using fins; and are cold blooded poikilothermic organisms. There are exceptions to these generalisations, e.g., some species of eels have no fins, lampreys and hagfish have no scales and some shark and pelagic predators are partially warm blooded. Fishes vary in size more than any other vertebrate groups. The world’s largest fish is the whale shark (Rhincodon typus), which can grow to 20 m long and a massive 34 t in weight. The smallest is arguably Paedocypris progenetica, part of the carp family, measuring only 7.9 mm long. Generated on 07/10/2021 https://marinewaters.fish.wa.gov.au/resource/fish-anatomy/ Page 1 of 18 Figure 1. The world’s largest fish – the whale shark (Rhincodon typus) There are three main groups (or classes) of fishes: 1. bony fish, 2. cartilaginous fish, and 3. jawless fish. Bony fish (Class Osteichthyes) Bony fish represent the largest and most diverse class of fishes, with well over 20,000 species. As the name suggests, bony fish have a skeleton made from bone. -

Teleost Fishes (The Teleostei)
OEB 130: BIOLOGY OF FISHES Lecture 4: Overview of ray-finned fish diversity (Actinopterygii) Announcements 1 1. Please review the syllabus for reading and lab information! 2. Please do the readings: for this week posted now. 3. Lab sections: 4. i) Dylan Wainwright, Thursday 2 - 4/5 pm ii) Kelsey Lucas, Friday 2 - 4/5 pm iii) Labs are in the Northwest Building basement (room B141) Please be on time at 2pm! 4. Lab sections done: first lab this week tomorrow! 5. First lab reading: Agassiz fish story; lab will be a bit shorter 6. Office hours to come Announcements 2 8 pages of general information on fish external anatomy and characters to help you learn some basic external fish anatomy and terminology – the last slides in the uploaded lecture Powerpoint file for today. Please look at these before lab this week, but no need to bring them in with you. Scanned from: Hastings, P. A., Walker, H. J. and Galland, G. R. (2014). Fishes: A guide to their diversity: Univ. of California Press. Next Monday: prepare to draw/diagram in lecture (colored pencils/pens will be useful) Lecture outline Lecture outline: 1. Brief review of the phylogeny and key traits so far 2. Actinopterygian clade: overview and introduction to key groups and selected key traits 3. Special focus on: 1. Fin ray structure and function 2. Lung and swimblader evolution 3. Early diversity of teleost fishes Selected key shared derived characters (synapomorphies) Review from last lecture Chondrichthyes (sharks and rays and ratfishes): 1. Dentition: multiple rows of unattached teeth 2. -

Unit 2. Fish Ecology Lesson 1
FISH ECOLOGY Unit 2. Fish Ecology Lesson 1. --What is a Fish? Lesson objectives: Students will understand external fish anatomy, and that fish come in many shapes and sizes The students will be able to identify the different zones of the ocean Students will become familiar with the methods that are used to study fish are diverse, and each has a purpose. Vocabulary words: vertebrae, planktonic, nektonic, benthic, continental margin, and many more pertaining to ocean zonation What is a Fish? A fish is defined as an aquatic lamprey and hagfish; and the or marine animal with shark, ray, chimaera, lungfish, vertebrae. All fish have and bony fishes. The bony vertebra, except sharks and fishes are the most common. A rays that have cartilage. bony fish has jaws that are well Cartilage is more flexible than developed, formed by true bone bone, but strong enough to rather than cartilage. Fish are support the body. They usually very different in appearance, possess gills in the adult stage size and shape. This all and have limbs in the form of depends on the environment fins. Fishes also include the that it lives in. jawless vertebrates such as the ©PROJECT OCEANOGRAPHY FALL 1999 FISH ECOLOGY 17 FISH ECOLOGY Fish Habitats Fish occupy almost every not associated with land) realm conceivable aquatic habitat. of the ocean. Describing the ocean is difficult, as there are many words that Geographic zonation defines are used to explain the land the coastline, and submerged and water realms. These land. Scientists use these habitats are defined by the words to help in the critters that live in each. -

Fish Dissection and Assays for Parasites
Center for Biodiversity and Conservation Network of Conservation Educators and Practitioners Parasite Biodiversity: Fish Dissection and Assays for Parasites Author(s): Danielle C. Claar, Armand Kuris, Katie L. Leslie, Rachel L. Welicky, Maureen A. Williams, and Chelsea L. Wood Source: Lessons in Conservation, Vol. 11, Issue 1, pp. 58-67 Published by: Network of Conservation Educators and Practitioners, Center for Biodiversity and Conservation, American Museum of Natural History Stable URL: ncep.amnh.org/linc This article is featured in Lessons in Conservation, the offcial journal of the Network of Conservation Educators and Practitioners (NCEP). NCEP is a collaborative project of the American Museum of Natural History’s Center for Biodiversity and Conservation (CBC) and a number of institutions and individuals around the world. Lessons in Conservation is designed to introduce NCEP teaching and learning resources (or “modules”) to a broad audience. NCEP modules are designed for undergraduate and professional level education. These modules—and many more on a variety of conservation topics—are available for free download at our website, ncep. amnh.org. Note to educators: access presentations, teaching notes, exercise solutions, and associated fles for these modules by registering as an educator, and searching for module by title. To learn more about NCEP, visit our website: ncep.amnh.org. All reproduction or distribution must provide full citation of the original work and provide a copyright notice as follows: “Copyright 2021, by the authors of the material and the Center for Biodiversity and Conservation of the American Museum of Natural History. All rights reserved.” Illustrations obtained from the American Museum of Natural History’s library: images.library.amnh.org/digital/ 58 EXERCISE Parasite Biodiversity: Fish Dissection and Assays for Parasites Danielle C. -

Fish Anatomy Coelacanth Bull Shark
Fish Anatomy Coelacanth Bull Shark NOVA Activity Ancient Creature of the Deep Coelacanths are difficult to classify. They have many characteristics in common with sharks, and yet in certain characteristics they more closely resemble other Moray Eel types of fish. In this activity, you will decide which type of fish—moray eels or bull sharks—is more closely related to coelacanths. Questions Procedure Write your answers on a separate sheet of paper. 1 Begin by reading about fish anatomy in your Basic Fish 1 What characteristics do all fishes have in common? Facts information below. 2 What characteristics of a coelacanth cause it to be 2 Use the Internet and print resources to learn more classified as a fish? about these three types of fish. Compare: 3 What features do the coelacanth and the bull shark • their skeletal types have in common? • their body coverings • whether they bear live young 4 Which features of a coelacanth are similar to those of • their buoyancy systems a moray eel? • whether they have gill slits or flaps 5 Do you think a coelacanth is more closely related to a • any other characteristics you learn about bull shark or a moray eel? Explain your reasoning. 3 On a separate sheet of paper, draw a coelacanth, bull shark, and moray eel and label the body parts of each. Basic Fish Facts DORSAL Fins: Help a fish move. The top fins are called dorsal fins. Dorsal Fin If there are two dorsal fins, the one nearest the head is called the first dorsal fin and the one behind it is the Pectoral Fin Caudal Fin second dorsal fin.