Fish Dissection and Assays for Parasites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
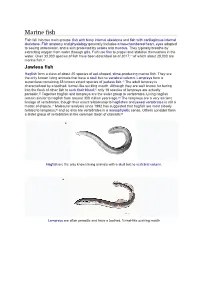
Marine Fish Fish Fall Into Two Main Groups: Fish with Bony Internal Skeletons and Fish with Cartilaginous Internal Skeletons
Marine fish Fish fall into two main groups: fish with bony internal skeletons and fish with cartilaginous internal skeletons. Fish anatomy and physiology generally includes a two-chambered heart, eyes adapted to seeing underwater, and a skin protected by scales and mucous. They typically breathe by extracting oxygen from water through gills. Fish use fins to propel and stabilise themselves in the water. Over 33,000 species of fish have been described as of 2017,[1] of which about 20,000 are marine fish.[2] Jawless fish Hagfish form a class of about 20 species of eel-shaped, slime-producing marine fish. They are the only known living animals that have a skull but no vertebral column. Lampreys form a superclass containing 38 known extant species of Jawless fish.[3] The adult lamprey is characterized by a toothed, funnel-like sucking mouth. Although they are well known for boring into the flesh of other fish to suck their blood,[4] only 18 species of lampreys are actually parasitic.[5] Together hagfish and lampreys are the sister group to vertebrates. Living hagfish remain similar to hagfish from around 300 million years ago.[6] The lampreys are a very ancient lineage of vertebrates, though their exact relationship to hagfishes and jawed vertebrates is still a matter of dispute.[7] Molecular analysis since 1992 has suggested that hagfish are most closely related to lampreys,[8] and so also are vertebrates in a monophyletic sense. Others consider them a sister group of vertebrates in the common taxon of craniata.[9] • Hagfish are the only known living animals with a skull but no vertebral column. -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

Explore a Fish Lesson Plan
Grade Level 4 - 12 EXPLORE A FISH Duration FISH DISSECTION 1 - 1 ½ hours Subject/Subject Area Focus Overview Science, language arts; Students will examine the external and internal anatomy of various fish Analysis, application, species. They will note similarities and differences. Students will then communication, use their observations to make inferences about the relationships among comparing similarities and them. differences, description, discussion, drawing, small Background information group work, using time This activity gives students first-hand experience exploring the and space, writing. adaptations which allow fish to function in their environment. Students look at both form and function of different systems to help understand Materials how specific adaptations assist organisms in adapting to their For each pair (or group) of environment. How do fish move through the water and keep their vertical students: position within the water? Students can make comparisons between their • Whole body fresh fish own anatomy and the anatomy of a fish. • Dissecting trays or thick pads of newspaper It is important for students to understand the purpose of this activity • Scissors or Scalpel is to study the internal and external anatomy of a fish. It requires (Most cuts can be concentration, listening skills and being able to follow directions. All made with a pair of students should be given the option of not participating in the activity classroom scissors) and be allowed an alternate activity. You may want to do a practice run on • Probe (A large partially your own. straightened paper clip works well) You can either do this activity as a teacher-led class discussion or break • Forceps (A nice tool but the students into cooperative learning groups and give each group: a fish, not necessary) “Explore a Fish” worksheet, dissection and anatomy sheet, newspaper • Paper towels and and something to cut the fish with. -

The Mouth and Gastrointestinal Tract of the African Lung Fish Protopterus Annectens (Owen 1839) in River Niger at Agenebode, Edo State Nigeria
Egyptian Journal of Aquatic Biology & Fisheries Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt. ISSN 1110 – 6131 Vol. 23(4): 181- 188 (2019) www.ejabf.journals.ekb.eg The mouth and gastrointestinal tract of the african lung fish Protopterus annectens (owen 1839) in River Niger at Agenebode, Edo State Nigeria Agbugui Marian Onwude1* and Oniye Sunnie Joshua2 1-Department of Biological Sciences, Edo University Iyamho, Edo State, Nigeria 2-Department of Zoology, Ahmadu Bello University, Zaria, Kaduna State, Nigeria *Corresponding Author: [email protected] ARTICLE INFO ABSTRACT Article History: The mouth of P. annectens is terminal, its jaws are modified to hold Received: May 24, 2019 incisors -scissor-like teeth that can hold and tear deep into the flesh of prey. Accepted: Sept. 28, 2019 The mouth opens up to 10% of its total body length which allows the fish to Online: Oct. 2019 seize its prey easily. The gastrointestinal (GIT) is straight, short and a _______________ composite comprising of many organs and tissues. The organs are wrapped in serosa. The morphology and histology of the GIT, (oesophagus to the cloaca) Keywords: reveals the adaptation to the food and feeding habit of fish. The numerous Protopterus annectens African lung fish mucosal folds in the intestine are a usual occurrence in carnivorous species. River Niger The ability of the intestine to distend and the presence of the numerous large Agenebode folds allow fishes to accommodate a large quantity of food items as digestion Gastrointestinal tract takes place and nutrients are being ingested. INTRODUCTION The West African Lung fish are an important source of food supply and protein for man. -

Fact Sheet: Fish Anatomy
Fact Sheet: Fish Anatomy WA Curriculum K-10 Science, SS Biology – ATAR, SS Biology – General, SS Integrated Science – ATAR, SS Integrated Science – General Region North Coast, Gascoyne Coast, West Coast, South Coast, Indian Ocean Territories Summary Fishes are a large and varied group of aquatic animals. Worldwide, there are over 32,000 described species, with over 4,400 in Australia (Australian Museum). They come in an amazing variety of shapes and sizes. All fishes have the following features in common: they live in water; have a backbone (vertebrate); and breathe using gills. Most fishes also: have scales; move using fins; and are cold blooded poikilothermic organisms. There are exceptions to these generalisations, e.g., some species of eels have no fins, lampreys and hagfish have no scales and some shark and pelagic predators are partially warm blooded. Fishes vary in size more than any other vertebrate groups. The world’s largest fish is the whale shark (Rhincodon typus), which can grow to 20 m long and a massive 34 t in weight. The smallest is arguably Paedocypris progenetica, part of the carp family, measuring only 7.9 mm long. Generated on 07/10/2021 https://marinewaters.fish.wa.gov.au/resource/fish-anatomy/ Page 1 of 18 Figure 1. The world’s largest fish – the whale shark (Rhincodon typus) There are three main groups (or classes) of fishes: 1. bony fish, 2. cartilaginous fish, and 3. jawless fish. Bony fish (Class Osteichthyes) Bony fish represent the largest and most diverse class of fishes, with well over 20,000 species. As the name suggests, bony fish have a skeleton made from bone. -

Teleost Fishes (The Teleostei)
OEB 130: BIOLOGY OF FISHES Lecture 4: Overview of ray-finned fish diversity (Actinopterygii) Announcements 1 1. Please review the syllabus for reading and lab information! 2. Please do the readings: for this week posted now. 3. Lab sections: 4. i) Dylan Wainwright, Thursday 2 - 4/5 pm ii) Kelsey Lucas, Friday 2 - 4/5 pm iii) Labs are in the Northwest Building basement (room B141) Please be on time at 2pm! 4. Lab sections done: first lab this week tomorrow! 5. First lab reading: Agassiz fish story; lab will be a bit shorter 6. Office hours to come Announcements 2 8 pages of general information on fish external anatomy and characters to help you learn some basic external fish anatomy and terminology – the last slides in the uploaded lecture Powerpoint file for today. Please look at these before lab this week, but no need to bring them in with you. Scanned from: Hastings, P. A., Walker, H. J. and Galland, G. R. (2014). Fishes: A guide to their diversity: Univ. of California Press. Next Monday: prepare to draw/diagram in lecture (colored pencils/pens will be useful) Lecture outline Lecture outline: 1. Brief review of the phylogeny and key traits so far 2. Actinopterygian clade: overview and introduction to key groups and selected key traits 3. Special focus on: 1. Fin ray structure and function 2. Lung and swimblader evolution 3. Early diversity of teleost fishes Selected key shared derived characters (synapomorphies) Review from last lecture Chondrichthyes (sharks and rays and ratfishes): 1. Dentition: multiple rows of unattached teeth 2. -

Unit 2. Fish Ecology Lesson 1
FISH ECOLOGY Unit 2. Fish Ecology Lesson 1. --What is a Fish? Lesson objectives: Students will understand external fish anatomy, and that fish come in many shapes and sizes The students will be able to identify the different zones of the ocean Students will become familiar with the methods that are used to study fish are diverse, and each has a purpose. Vocabulary words: vertebrae, planktonic, nektonic, benthic, continental margin, and many more pertaining to ocean zonation What is a Fish? A fish is defined as an aquatic lamprey and hagfish; and the or marine animal with shark, ray, chimaera, lungfish, vertebrae. All fish have and bony fishes. The bony vertebra, except sharks and fishes are the most common. A rays that have cartilage. bony fish has jaws that are well Cartilage is more flexible than developed, formed by true bone bone, but strong enough to rather than cartilage. Fish are support the body. They usually very different in appearance, possess gills in the adult stage size and shape. This all and have limbs in the form of depends on the environment fins. Fishes also include the that it lives in. jawless vertebrates such as the ©PROJECT OCEANOGRAPHY FALL 1999 FISH ECOLOGY 17 FISH ECOLOGY Fish Habitats Fish occupy almost every not associated with land) realm conceivable aquatic habitat. of the ocean. Describing the ocean is difficult, as there are many words that Geographic zonation defines are used to explain the land the coastline, and submerged and water realms. These land. Scientists use these habitats are defined by the words to help in the critters that live in each. -

1.0 Anatomy and Physiology of Salmonids
Canada Department of Fisheries and Oceans AnimalUser Training Template 1.0 Anatomy and Physiology of Salmonids 1.1 Introduction: This template is intended for use by instructors to train the Department of Fisheries and Oceans (DFO) staff and students in the anatomy and physiology of salmonids. Templates are used to provide the minimum requirements necessary in a training exercise, but the instructor may add additional material. An experienced instructor must demonstrate the methods outlined in this template. Handson training of staff is a requirement for facility approval by the Canadian Council on Animal Care, of which DFO is a member. This template is part of a comprehensive DFO Science Branch series on training for users of aquatic research animals. 1.2 Rationale: There are over 50 species of fish and shellfish used as laboratory animals by the scientific community in Canada alone. Salmonid species are among the most common laboratory animals and include but are not limited to: Rainbow trout, Cutthroat trout, Atlantic salmon, Brook trout, Steelhead salmon, Brown trout, Arctic char, Lake trout, Chum salmon, Sockeye salmon, Pink salmon, Coho salmon and Chinook salmon. In order to properly identify abnormalities and signs of disease or distress in fish maintained for the purposes of scientific study, it is important to recognize and understand the basic anatomy and physiology of the species being studied. An understanding of anatomy is critical and ensures proper and humane methods are used for procedures such as blood sampling and tagging or marking with minimal longterm effect on the fish. 1.3 Authority: The staff, consultant veterinarian or Animal Care Committee is responsible for providing information about anatomy and physiology of the fish species used for scientific study in their respective regions. -

Fish Anatomy Coelacanth Bull Shark
Fish Anatomy Coelacanth Bull Shark NOVA Activity Ancient Creature of the Deep Coelacanths are difficult to classify. They have many characteristics in common with sharks, and yet in certain characteristics they more closely resemble other Moray Eel types of fish. In this activity, you will decide which type of fish—moray eels or bull sharks—is more closely related to coelacanths. Questions Procedure Write your answers on a separate sheet of paper. 1 Begin by reading about fish anatomy in your Basic Fish 1 What characteristics do all fishes have in common? Facts information below. 2 What characteristics of a coelacanth cause it to be 2 Use the Internet and print resources to learn more classified as a fish? about these three types of fish. Compare: 3 What features do the coelacanth and the bull shark • their skeletal types have in common? • their body coverings • whether they bear live young 4 Which features of a coelacanth are similar to those of • their buoyancy systems a moray eel? • whether they have gill slits or flaps 5 Do you think a coelacanth is more closely related to a • any other characteristics you learn about bull shark or a moray eel? Explain your reasoning. 3 On a separate sheet of paper, draw a coelacanth, bull shark, and moray eel and label the body parts of each. Basic Fish Facts DORSAL Fins: Help a fish move. The top fins are called dorsal fins. Dorsal Fin If there are two dorsal fins, the one nearest the head is called the first dorsal fin and the one behind it is the Pectoral Fin Caudal Fin second dorsal fin. -

Betta Fish Evolutionary Morphology
Betta Fish Evolutionary Morphology Shijie He, CongCheng Yan, Chutian Chen, Chengchao Jin, Wancheng Chen 1. Introduction 2. Data 3. Image Analysis a. Color Calibration Content b. Fish Segmentation c. Feature Extraction 4. Genetic Mapping 5. Conclusion and Future Work 1.Introduction Introduction Goal: Figure out how evolution can affect the traits of Betta fish. The project is consisted of image analysis and genetic mapping. Image Analysis: 1. Read raw data (CR3 pictures) 2. Color calibration (self-designed algorithms) 3. Segmentation (self-designed algorithms) 4. Fish Anatomy and feature extraction (transfer learning) Genetic Mapping: 1. Utilize the final output from image analysis part as input 2. Mapping genetic data with features of fish Workflow of project 2. Data Data Data Description: ● The data of the project is Betta fish pictures. There are pure blue or red fishes and also many descendant fishes which mixed blue and red colors in different degree. ● Obtained 331 fishes and 14,909 pictures in total Data Input: Remove the influence of the automatic adjustment to the photos, restore real color, make analysis more accurate Methods: ● Use raw images (.CR3 images) instead of .JPG images ● Convert .CR3 to .tiff with package “Rawpy”, transform the images into RGB data Data Raw Image JPG Image 3. Image Analysis a. Color Calibration Before Calibration After Calibration Based on the color cards on the images, calibrate the color of the images according to the standard color card. Steps: 1. Extract the color cards 2. Detect the color patches in the cards 3. Match the color patches to the standard color 4. -

Fish Sense Fish Depend on Their Senses for Survival
Chapter 2 • Lesson 1 Fish Sense Fish depend on their senses for survival. Whether they eat a meal or become a meal depends on their ability to see, hear, smell, taste, and detect vibrations. © 2010 Minnesota DNR • MinnAqua • USFWS Sport Fish Restoration 2:1-B Chapter 2 • Lesson 1 • Fish Sense Table of Contents Fish Sense ............................................................................2:1-A Minnesota Academic Standards ............................................... 2:1-C Environmental Literacy Scope and Sequence .......................... 2:1-C Instructor’s Background Information .....................................2:1-1-4 Summary ................................................................................... 2:1-1 Student Objectives .................................................................... 2:1-1 Materials .................................................................................... 2:1-1 Activity ...................................................................................... 2:1-5 Procedure ................................................................................... 2:1-5 Assessment Options .................................................................. 2:1-9 Checklist .............................................................................. 2:1-10 Scoring Rubric ..................................................................... 2:1-11 Extensions .............................................................................. 2:1-12 K-2 Option ............................................................................. -

An At-Home Lesson for Grades 6-8 Produced By
Florida's Fintastic Sharks and Rays An at-home lesson for grades 6-8 Produced by: 1 What are sharks and rays? Believe it or not, they’re a type of fish! When you think “fish”, you probably picture a trout or tuna—something you’d commonly see on the end of a hook or on a plate. But fishes come in all shapes and sizes, some with saxophone shaped bodies (seahorses!) or elongated like a snake (eels!). No matter the look, all fishes share the following key characteristics that classify them into this vertebrate group. So before we get into sharks and rays, let’s start with a more general overview that applies all types of fishes. While we’re at it, what is the correct plural version of the word “fish”? Well, it depends. If you are talking about more than one individual fish, the correct plural form is “fish” (I caught a bunch of fish today.). However, when scientists talk about multiple different types or groups of fish, the plural is “fishes” (My research focuses on coral reef fishes.) Fishes have the simplest of vertebrate hearts with only two chambers- one atrium and one ventricle. The atrium brings in de-oxygenated blood from the body, pumps it to the ventricle which sends blood to the Gills. Blood is oxygenated through the vessels in the gills and circulated throughout the body. The vertebral column in a fish runs down the middle of its back in a similar manner to humans. Not all fishes have bony vertebrae. Fishes are cold-blooded, or ectothermic.