A Standardized Ultrasonography Classification for Channel Catfish Ovarian Development Noel D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume III, Chapter 9 Channel Catfish
Volume III, Chapter 9 Channel Catfish TABLE OF CONTENTS 9.0 Channel Catfish (Ictalurus punctatus) ........................................................................ 9-1 9.1 Introduction................................................................................................................. 9-1 9.2 Life History & Requirements...................................................................................... 9-2 9.2.1 Spawn Timing & Conditions................................................................................ 9-2 9.2.2 Incubation ............................................................................................................ 9-3 9.2.3 Larvae & Juveniles .............................................................................................. 9-3 9.2.4 Adult..................................................................................................................... 9-4 9.2.5 Movements ........................................................................................................... 9-5 9.3 Status & Abundance.................................................................................................... 9-5 9.3.1 Abundance............................................................................................................ 9-5 9.3.2 Productivity.......................................................................................................... 9-5 9.3.3 Supplementation.................................................................................................. -

Channel Catfish Life History and Biology
SRAC Publication No. 180 Southern Regional Aquaculture Center December, 1988 . Channel Catfish Life History and Biology Thomas L. Wellborn* Channel cattish, Ictalurus punctatus Rocky Mountains. Since then chan- is located on the back between the (Rafinesque), is the most important nel catfish have been widely intro- dorsal and caudal fins (Fig. 1). One species of aquatic animal commer- duced throughout the United States conspicuous characteristic of all cially cultured in the United States. and the world. catfish is the presence of barbels It belongs to the family Ictaluridae, around the mouth. The barbels are order Siluriformes. Members of the Physical characteristics arranged in a definite pattern with order Siluriformes are found in fresh Like all native North American cat- four under the jaw and one on each and salt water worldwide. There are fishes, a channel catfish has a body tip of the maxilla (upper jaw). at least 39 species of catfish in North that is cylindrical in cross-section, America, but only six have been cul- and lacks scales. Fins are soft-rayed The channel catfish is the only tured or have potential for commer- except for the dorsal and pectoral spotted North American catfish with cial production. They are the blue fins which have sharp, hard spines a deeply forked tail. There are 24-29 catfish, Ictalurus furcatus (LeSueur); that can inflict a nasty, painful rays in the anal fin. They are general- the white catfish, Ictalurus catus wound if a catfish is handled care- ly olivaceous to blue on the back, (Linnaeus); the black bullhead, Ic- lessly. -

Full Text in Pdf Format
Vol. 1: 117–132, 2015 SEXUALITY AND EARLY DEVELOPMENT IN AQUATIC ORGANISMS Published online June 11 doi: 10.3354/sedao00012 Sex Early Dev Aquat Org OPENPEN ACCESSCCESS Sexual development and maturity scale for the angel shark Squatina squatina (Elasmobranchii: Squatinidae), with comments on the adequacy of general maturity scales Filip Osaer1,2,3,*, Krupskaya Narváez1,2,3, José G. Pajuelo2, José M. Lorenzo2 1ELASMOCAN, Asociación Canaria para la Investigación y Conservación de los Elasmobranquios, 35001 Las Palmas de Gran Canaria, Spain 2Departamento de Biología, Universidad de Las Palmas de Gran Canaria, Edificio de Ciencias Básicas, Campus de Tafira, 35017 Las Palmas de Gran Canaria, Spain 3Fundación Colombiana para la Investigación y Conservación de Tiburones y Rayas, SQUALUS, Carrera 60A No 11−39, Cali, Colombia ABSTRACT: This paper contributes to the reproductive biology of the genus Squatina and aims to complement the criteria, uniformity and adaptable staging of sexual maturity scales for elasmo- branchs based on data from the angel shark S. squatina captured near the island of Gran Canaria (Canary Islands, Central-East Atlantic). Both sexes presented a paired reproductive tract with both sides active and asymmetric gonad development. Microscopic and macroscopic observations of the testes were consistent and indicated seasonality of spermatogenesis. The spermatocyst de - velopment pattern in mature individuals could not be assigned to any of the categories described in the literature. The ovaries−epigonal organ association was of the external type. Although all Squatinidae share a conservative morphology, they show differences across species in the func- tionality of the paired reproductive tract, seasonality of spermatogenesis, coiled spermatozoa and the presence of egg candles. -

A Draft Genome of the Striped Catfish, Pangasianodon Hypophthalmus, for Comparative Analysis of Genes Relevant to Development An
Kim et al. BMC Genomics (2018) 19:733 https://doi.org/10.1186/s12864-018-5079-x RESEARCHARTICLE Open Access A draft genome of the striped catfish, Pangasianodon hypophthalmus, for comparative analysis of genes relevant to development and a resource for aquaculture improvement Oanh T. P. Kim1*† , Phuong T. Nguyen1†, Eiichi Shoguchi2†, Kanako Hisata2, Thuy T. B. Vo1, Jun Inoue2, Chuya Shinzato2,4, Binh T. N. Le1, Koki Nishitsuji2, Miyuki Kanda3, Vu H. Nguyen1, Hai V. Nong1 and Noriyuki Satoh2* Abstract Background: The striped catfish, Pangasianodon hypophthalmus, is a freshwater and benthopelagic fish common in the Mekong River delta. Catfish constitute a valuable source of dietary protein. Therefore, they are cultured worldwide, and P. hypophthalmus is a food staple in the Mekong area. However, genetic information about the culture stock, is unavailable for breeding improvement, although genetics of the channel catfish, Ictalurus punctatus, has been reported. Toacquiregenomesequencedataasausefulresourcefor marker-assisted breeding, we decoded a draft genome of P. hypophthalmus and performed comparative analyses. Results: Using the Illumina platform, we obtained both nuclear and mitochondrial DNA sequences. Molecular phylogeny using the mitochondrial genome confirmed that P. hypophthalmus is a member of the family Pangasiidae and is nested within a clade including the families Cranoglanididae and Ictaluridae. The nuclear genome was estimated at approximately 700 Mb, assembled into 568 scaffolds with an N50 of 14.29 Mbp, and was estimated to contain ~ 28,600 protein-coding genes, comparable to those of channel catfish and zebrafish. Interestingly, zebrafish produce gadusol, but genes for biosynthesis of this sunscreen compound have been lost from catfish genomes. -

Reproductive Biology of the Bonnethead (Sphyrna Tiburo) from the Southeastern U.S
University of North Florida UNF Digital Commons UNF Graduate Theses and Dissertations Student Scholarship 2014 Reproductive Biology of the Bonnethead (Sphyrna tiburo) from the Southeastern U.S. Atlantic Coast Melissa I. Gonzalez De Acevedo University of North Florida, [email protected] Follow this and additional works at: https://digitalcommons.unf.edu/etd Part of the Biology Commons, and the Ecology and Evolutionary Biology Commons Suggested Citation Gonzalez De Acevedo, Melissa I., "Reproductive Biology of the Bonnethead (Sphyrna tiburo) from the Southeastern U.S. Atlantic Coast" (2014). UNF Graduate Theses and Dissertations. 534. https://digitalcommons.unf.edu/etd/534 This Master's Thesis is brought to you for free and open access by the Student Scholarship at UNF Digital Commons. It has been accepted for inclusion in UNF Graduate Theses and Dissertations by an authorized administrator of UNF Digital Commons. For more information, please contact Digital Projects. © 2014 All Rights Reserved REPRODUCTIVE BIOLOGY OF THE BONNETHEAD (SPHYRNA TIBURO) FROM THE SOUTHEASTERN U.S. ATLANTIC COAST by Melissa Gonzalez De Acevedo A thesis submitted to the Department of Biology in partial fulfillment of the requirements for the degree of Masters of Science in Biology UNIVERSITY OF NORTH FLORIDA COLLEGE OF ARTS AND SCIENCES December 2014 Unpublished work, © Melissa Gonzalez De Acevedo CERTIFICATE OF APPROVAL The thesis “Reproductive biology of the bonnethead (Sphyrna tiburo) from the southeastern U.S. Atlantic coast” submitted by Melissa Gonzalez De Acevedo Approved by the thesis committee: Date Dr. Jim Gelsleichter Committee Chair Dr. Carolyn Belcher Dr. Eric Johnson Accepted for the Department of Biology: Dr. Cliff Ross Assistant Chair Accepted for the College of Arts and Sciences: Dr. -
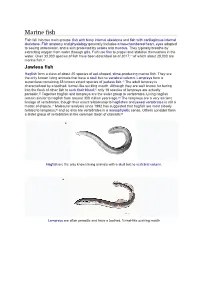
Marine Fish Fish Fall Into Two Main Groups: Fish with Bony Internal Skeletons and Fish with Cartilaginous Internal Skeletons
Marine fish Fish fall into two main groups: fish with bony internal skeletons and fish with cartilaginous internal skeletons. Fish anatomy and physiology generally includes a two-chambered heart, eyes adapted to seeing underwater, and a skin protected by scales and mucous. They typically breathe by extracting oxygen from water through gills. Fish use fins to propel and stabilise themselves in the water. Over 33,000 species of fish have been described as of 2017,[1] of which about 20,000 are marine fish.[2] Jawless fish Hagfish form a class of about 20 species of eel-shaped, slime-producing marine fish. They are the only known living animals that have a skull but no vertebral column. Lampreys form a superclass containing 38 known extant species of Jawless fish.[3] The adult lamprey is characterized by a toothed, funnel-like sucking mouth. Although they are well known for boring into the flesh of other fish to suck their blood,[4] only 18 species of lampreys are actually parasitic.[5] Together hagfish and lampreys are the sister group to vertebrates. Living hagfish remain similar to hagfish from around 300 million years ago.[6] The lampreys are a very ancient lineage of vertebrates, though their exact relationship to hagfishes and jawed vertebrates is still a matter of dispute.[7] Molecular analysis since 1992 has suggested that hagfish are most closely related to lampreys,[8] and so also are vertebrates in a monophyletic sense. Others consider them a sister group of vertebrates in the common taxon of craniata.[9] • Hagfish are the only known living animals with a skull but no vertebral column. -

Monoculture of Channel Catfish in Farm Ponds
AQUAGUIDE MISSOURI DEPARTMENT OF CONSERVATION Monoculture of Channel Catfish in Farm Ponds Channel catfish, one of Missouri’s most popular to spawn as the water temperature reaches 75 to 80 sport and food fish, have been stocked in ponds degrees. When this happens, the male will seek out throughout the state. Many small ponds are managed and prepare a nest site in a secluded, semi-dark area. exclusively for channel catfish. The female spawns only once per year, though she will A small, well-managed pond can produce 300 to 500 sometimes pair before she is ready to spawn. After pounds of catfish per surface acre each year, providing spawing, the eggs usually take eight days to hatch and many hours of recreation and an abundance of high another eight days for the fry to prepare to leave the nest. quality food for the table. During this time, the male will first protect the eggs, then Channel catfish have scaleless, cylindrical bodies the fry until they leave the nest. with soft fins, except for the dorsal and pectoral fins, which have hard, sharp spines that can inflict a painful wound. A catfish’s color varies, depending on water Ponds for Catfish clarity. In clear water, catfish may appear dark blue or Ponds suitable for exclusive channel catfish black, while in muddy water they may be a light yellow production should be at least eight feet deep with pond or gray. Young channel catfish are typically spotted. edges sloping quickly to three feet deep to reduce Channel catfish eat a variety of both plant and aquatic vegetation problems. -

2021 Fish Suppliers
2021 Fish Suppliers A.B. Jones Fish Hatchery Largemouth bass, hybrid bluegill, bluegill, black crappie, triploid grass carp, Nancy Jones gambusia – mosquito fish, channel catfish, bullfrog tadpoles, shiners 1057 Hwy 26 Williamsburg, KY 40769 (606) 549-2669 ATAC, LLC Pond Management Specialist Fathead minnows, golden shiner, goldfish, largemouth bass, smallmouth bass, Rick Rogers hybrid bluegill, bluegill, redear sunfish, walleye, channel catfish, rainbow trout, PO Box 1223 black crappie, triploid grass carp, common carp, hybrid striped bass, koi, Lebanon, OH 45036 shubunkin goldfish, bullfrog tadpoles, and paddlefish (513) 932-6529 Anglers Bait-n-Tackle LLC Fathead minnows, rosey red minnows, bluegill, hybrid bluegill, goldfish and Kaleb Rodebaugh golden shiners 747 North Arnold Ave Prestonsburg, KY 606-886-1335 Andry’s Fish Farm Bluegill, hybrid bluegill, largemouth bass, koi, channel catfish, white catfish, Lyle Andry redear sunfish, black crappie, tilapia – human consumption only, triploid grass 10923 E. Conservation Club Road carp, fathead minnows and golden shiners Birdseye, IN 47513 (812) 389-2448 Arkansas Pondstockers, Inc Channel catfish, bluegill, hybrid bluegill, redear sunfish, largemouth bass, Michael Denton black crappie, fathead minnows, and triploid grass carp PO Box 357 Harrisbug, AR 75432 (870) 578-9773 Aquatic Control, Inc. Largemouth bass, bluegill, channel catfish, triploid grass carp, fathead Clinton Charlton minnows, redear sunfish, golden shiner, rainbow trout, and hybrid striped bass 505 Assembly Drive, STE 108 -

Anatomy and Go Fish! Background
Anatomy and Go Fish! Background Introduction It is important to properly identify fi sh for many reasons: to follow the rules and regulations, for protection against sharp teeth or protruding spines, for the safety of the fi sh, and for consumption or eating purposes. When identifying fi sh, scientists and anglers use specifi c vocabulary to describe external or outside body parts. These body parts are common to most fi sh. The difference in the body parts is what helps distinguish one fi sh from another, while their similarities are used to classify them into groups. There are approximately 29,000 fi sh species in the world. In order to identify each type of fi sh, scientists have grouped them according to their outside body parts, specifi cally the number and location of fi ns, and body shape. Classifi cation Using a system of classifi cation, scientists arrange all organisms into groups based on their similarities. The fi rst system of classifi cation was proposed in 1753 by Carolus Linnaeus. Linnaeus believed that each organism should have a binomial name, genus and species, with species being the smallest organization unit of life. Using Linnaeus’ system as a guide, scientists created a hierarchical system known as taxonomic classifi cation, in which organisms are classifi ed into groups based on their similarities. This hierarchical system moves from largest and most general to smallest and most specifi c: kingdom, phylum, class, order, family, genus, and species. {See Figure 1. Taxonomic Classifi cation Pyramid}. For example, fi sh belong to the kingdom Animalia, the phylum Chordata, and from there are grouped more specifi cally into several classes, orders, families, and thousands of genus and species. -

Petition to List US Populations of Lake Sturgeon (Acipenser Fulvescens)
Petition to List U.S. Populations of Lake Sturgeon (Acipenser fulvescens) as Endangered or Threatened under the Endangered Species Act May 14, 2018 NOTICE OF PETITION Submitted to U.S. Fish and Wildlife Service on May 14, 2018: Gary Frazer, USFWS Assistant Director, [email protected] Charles Traxler, Assistant Regional Director, Region 3, [email protected] Georgia Parham, Endangered Species, Region 3, [email protected] Mike Oetker, Deputy Regional Director, Region 4, [email protected] Allan Brown, Assistant Regional Director, Region 4, [email protected] Wendi Weber, Regional Director, Region 5, [email protected] Deborah Rocque, Deputy Regional Director, Region 5, [email protected] Noreen Walsh, Regional Director, Region 6, [email protected] Matt Hogan, Deputy Regional Director, Region 6, [email protected] Petitioner Center for Biological Diversity formally requests that the U.S. Fish and Wildlife Service (“USFWS”) list the lake sturgeon (Acipenser fulvescens) in the United States as a threatened species under the federal Endangered Species Act (“ESA”), 16 U.S.C. §§1531-1544. Alternatively, the Center requests that the USFWS define and list distinct population segments of lake sturgeon in the U.S. as threatened or endangered. Lake sturgeon populations in Minnesota, Lake Superior, Missouri River, Ohio River, Arkansas-White River and lower Mississippi River may warrant endangered status. Lake sturgeon populations in Lake Michigan and the upper Mississippi River basin may warrant threatened status. Lake sturgeon in the central and eastern Great Lakes (Lake Huron, Lake Erie, Lake Ontario and the St. Lawrence River basin) seem to be part of a larger population that is more widespread. -

Oogenesis and Egg Quality in Finfish: Yolk Formation and Other Factors
fishes Review Oogenesis and Egg Quality in Finfish: Yolk Formation and Other Factors Influencing Female Fertility Benjamin J. Reading 1,2,*, Linnea K. Andersen 1, Yong-Woon Ryu 3, Yuji Mushirobira 4, Takashi Todo 4 and Naoshi Hiramatsu 4 1 Department of Applied Ecology, North Carolina State University, Raleigh, NC 27695, USA; [email protected] 2 Pamlico Aquaculture Field Laboratory, North Carolina State University, Aurora, NC 27806, USA 3 National Institute of Fisheries Science, Gijang, Busan 46083, Korea; [email protected] 4 Faculty of Fisheries Sciences, Hokkaido University, Minato, Hakodate, Hokkaido 041-8611, Japan; [email protected] (Y.M.); todo@fish.hokudai.ac.jp (T.T.); naoshi@fish.hokudai.ac.jp (N.H.) * Correspondence: [email protected]; Tel.: +1-919-515-3830 Received: 28 August 2018; Accepted: 16 November 2018; Published: 21 November 2018 Abstract: Egg quality in fishes has been a topic of research in aquaculture and fisheries for decades as it represents an important life history trait and is critical for captive propagation and successful recruitment. A major factor influencing egg quality is proper yolk formation, as most fishes are oviparous and the developing offspring are entirely dependent on stored egg yolk for nutritional sustenance. These maternally derived nutrients consist of proteins, carbohydrates, lipids, vitamins, minerals, and ions that are transported from the liver to the ovary by lipoprotein particles including vitellogenins. The yolk composition may be influenced by broodstock diet, husbandry, and other intrinsic and extrinsic conditions. In addition, a number of other maternal factors that may influence egg quality also are stored in eggs, such as gene transcripts, that direct early embryonic development. -

History of Fishes - Structural Patterns and Trends in Diversification
History of fishes - Structural Patterns and Trends in Diversification AGNATHANS = Jawless • Class – Pteraspidomorphi • Class – Myxini?? (living) • Class – Cephalaspidomorphi – Osteostraci – Anaspidiformes – Petromyzontiformes (living) Major Groups of Agnathans • 1. Osteostracida 2. Anaspida 3. Pteraspidomorphida • Hagfish and Lamprey = traditionally together in cyclostomata Jaws = GNATHOSTOMES • Gnathostomes: the jawed fishes -good evidence for gnathostome monophyly. • 4 major groups of jawed vertebrates: Extinct Acanthodii and Placodermi (know) Living Chondrichthyes and Osteichthyes • Living Chondrichthyans - usually divided into Selachii or Elasmobranchi (sharks and rays) and Holocephali (chimeroids). • • Living Osteichthyans commonly regarded as forming two major groups ‑ – Actinopterygii – Ray finned fish – Sarcopterygii (coelacanths, lungfish, Tetrapods). • SARCOPTERYGII = Coelacanths + (Dipnoi = Lung-fish) + Rhipidistian (Osteolepimorphi) = Tetrapod Ancestors (Eusthenopteron) Close to tetrapods Lungfish - Dipnoi • Three genera, Africa+Australian+South American ACTINOPTERYGII Bichirs – Cladistia = POLYPTERIFORMES Notable exception = Cladistia – Polypterus (bichirs) - Represented by 10 FW species - tropical Africa and one species - Erpetoichthys calabaricus – reedfish. Highly aberrant Cladistia - numerous uniquely derived features – long, independent evolution: – Strange dorsal finlets, Series spiracular ossicles, Peculiar urohyal bone and parasphenoid • But retain # primitive Actinopterygian features = heavy ganoid scales (external