Diagnostic Imaging of the Nasolacrimal Drainage System. Part
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ciliary Zonule Sclera (Suspensory Choroid Ligament)
ACTIVITIES Complete Diagrams PNS 18 and 19 Complete PNS 23 Worksheet 3 #1 only Complete PNS 24 Practice Quiz THE SPECIAL SENSES Introduction Vision RECEPTORS Structures designed to respond to stimuli Variable complexity GENERAL PROPERTIES OF RECEPTORS Transducers Receptor potential Generator potential GENERAL PROPERTIES OF RECEPTORS Stimulus causing receptor potentials Generator potential in afferent neuron Nerve impulse SENSATION AND PERCEPTION Stimulatory input Conscious level = perception Awareness = sensation GENERAL PROPERTIES OF RECEPTORS Information conveyed by receptors . Modality . Location . Intensity . Duration ADAPTATION Reduction in rate of impulse transmission when stimulus is prolonged CLASSIFICATION OF RECEPTORS Stimulus Modality . Chemoreceptors . Thermoreceptors . Nociceptors . Mechanoreceptors . Photoreceptors CLASSIFICATION OF RECEPTORS Origin of stimuli . Exteroceptors . Interoceptors . Proprioceptors SPECIAL SENSES Vision Hearing Olfaction Gustation VISION INTRODUCTION 70% of all sensory receptors are in the eye Nearly half of the cerebral cortex is involved in processing visual information Optic nerve is one of body’s largest nerve tracts VISION INTRODUCTION The eye is a photoreceptor organ Refraction Conversion (transduction) of light into AP’s Information is interpreted in cerebral cortex Eyebrow Eyelid Eyelashes Site where conjunctiva merges with cornea Palpebral fissure Lateral commissure Eyelid Medial commissure (a) Surface anatomy of the right eye Figure 15.1a Orbicularis oculi muscle -

Eyelid Conjunctival Tumors
EYELID &CONJUNCTIVAL TUMORS PHOTOGRAPHIC ATLAS Dr. Olivier Galatoire Dr. Christine Levy-Gabriel Dr. Mathieu Zmuda EYELID & CONJUNCTIVAL TUMORS 4 EYELID & CONJUNCTIVAL TUMORS Dear readers, All rights of translation, adaptation, or reproduction by any means are reserved in all countries. The reproduction or representation, in whole or in part and by any means, of any of the pages published in the present book without the prior written consent of the publisher, is prohibited and illegal and would constitute an infringement. Only reproductions strictly reserved for the private use of the copier and not intended for collective use, and short analyses and quotations justified by the illustrative or scientific nature of the work in which they are incorporated, are authorized (Law of March 11, 1957 art. 40 and 41 and Criminal Code art. 425). EYELID & CONJUNCTIVAL TUMORS EYELID & CONJUNCTIVAL TUMORS 5 6 EYELID & CONJUNCTIVAL TUMORS Foreword Dr. Serge Morax I am honored to introduce this Photographic Atlas of palpebral and conjunctival tumors,which is the culmination of the close collaboration between Drs. Olivier Galatoire and Mathieu Zmuda of the A. de Rothschild Ophthalmological Foundation and Dr. Christine Levy-Gabriel of the Curie Institute. The subject is now of unquestionable importance and evidently of great interest to Ophthalmologists, whether they are orbital- palpebral specialists or not. Indeed, errors or delays in the diagnosis of tumor pathologies are relatively common and the consequences can be serious in the case of malignant tumors, especially carcinomas. Swift diagnosis and anatomopathological confirmation will lead to a treatment, discussed in multidisciplinary team meetings, ranging from surgery to radiotherapy. -

Plastic Surgery for Pigmented Hairy Naevus of the Eyelids by Excision and Masquerade Skin Graft
Brit. J. Ophthal. (I969) 53, 343 Br J Ophthalmol: first published as 10.1136/bjo.53.5.343 on 1 May 1969. Downloaded from Plastic surgery for pigmented hairy naevus of the eyelids By excision and masquerade skin graft B. HIRSHOWITZ AND D. MAHLER Department of Plastic Surgery, Rambam Government Hospital, Haifa, Israel The simultaneous excision of a lesion affecting the skin of both upper and lower eyelids carries with it the problem of skin replacement. Both aesthetic and functional require- ments will have to be met in the reconstruction. Ectropion could complicate such a repair, and loss of eyelashes would add to the disfigurement. All these aspects had to be considered in a patient with a darkly pigmented hairy naevus involving both eyelids and the surrounding skin. Case report copyright. A 15-year-old boy had a deeply pigmented hairy naevus of the upper and lower eyelids on the left side. The naevus extended to both inner and outer canthi, the eyebrow, and the temporal and cheek regions (Fig. i). The ciliary borders of both eyelids were also involved. The operation was indicated because of severe psychological disturbances engendered by this disfigurement. http://bjo.bmj.com/ F_I. i Appearance before operation ......... ..... on September 26, 2021 by guest. Protected Operative technique The naevus was almost completely excised, apart from its extension into the eyebrow. Both upper and lower eyelashes were removed together with the naevus. Care was taken not to interfere with the lacrimal punctum. Immobilization of both eyelids was attained by a continuous wire suture running intratarsally. In effect, this adaptation of eyelid margins resulted in almost a complete tarsorrhaphy. -

New Theory on Facial Beauty: Ideal Dimensions in the Face and Its Application to Your Practice by Dr
New Theory on Facial Beauty: Ideal Dimensions in the Face And its application to your practice By Dr. Philip Young Aesthetic Facial Plastic Surgery 2015 Bellevue, Washington American Brazilian Aesthetic Meeting • Hello my presentation is on studying some further elements of a new theory on facial beauty called the Circles of Prominence. • Specifically we are going to be studying some key dimensions in the face that I think could possibly help your practice. • I’m from Bellevue Washington Home of Bill Gates, Microsoft and Starbucks. Beauty In my opinion Beauty is the most important trait that we have and it is the one trait that can have the most dramatic impact in our lives. Obviously finding the answer for Beauty is essential in our industry. The answers have alluded us: the magic number of Phi, cephalometrics, the neo classical canons by Leonardo Da Vinci, the averageness theory, etc. have all come short in finding what makes a face beautiful. • The Circles of Prominence is a theory that I discovered in 2003-2005 and was published in the Archives of Facial Plastic Surgery in 2006 and Received the Sir Harold Delf Gillies Award from the American Academy of Facial Plastic Surgery. The Circles of Prominence • Original published Archives FPS 2006 • Based on the idea that there is an ideal • Everything on the face has an ideal as well • Because we spend so much time looking at the iris • All dimensions of the face are related to the width of the iris • Obviously with a better definition of beauty our results in plastic surgery can be improved • The circles of prominence is based on the belief that there is an ideal. -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos. -

Acquired Etiologies of Lacrimal System Obstructions
5 Acquired Etiologies of Lacrimal System Obstructions Daniel P. Schaefer Acquired obstructions of the lacrimal excretory outfl ow system will produce the symptoms of epiphora, mucopurulent discharge, pain, dacryocystitis, and even cellulitis, prompting the patient to seek the ophthalmologist for evaluation and treatment. Impaired tear outfl ow may be functional, structural, or both. The causes may be primary – those resulting from infl ammation of unknown causes that lead to occlusive fi brosis—or secondary, resulting from infections, infl amma- tion, trauma, malignancies, toxicity, or mechanical causes. Secondary acquired dacryostenosis and obstruction may result from many causes, both common and obscure. Occasionally, the precise pathogenesis of nasolacrimal duct obstruction will, despite years of investigations, be elusive. To properly evaluate and appropriately treat the patient, the ophthal- mologist must have knowledge and comprehension of the lacrimal anatomy, the lacrimal apparatus, pathophysiology, ocular and nasal relationships, ophthalmic and systemic disease process, as well as the topical and systemic medications that can affect the nasolacrimal duct system. One must be able to assess if the cause is secondary to outfl ow anomalies, hypersecretion or refl ex secretion, pseudoepiphora, eyelid malposition abnormalities, trichiasis, foreign bodies and conjunctival concretions, keratitis, tear fi lm defi ciencies or instability, dry eye syn- dromes, ocular surface abnormalities, irritation or tumors affecting the trigeminal nerve, allergy, medications, or environmental factors. Abnormalities of the lacrimal pump function can result from involu- tional changes, eyelid laxity, facial nerve paralysis, or fl oppy eyelid syndrome, all of which displace the punctum from the lacrimal lake. If the cause is secondary to obstruction of the nasolacrimal duct system, the ophthalmologist must be able to determine where the anomaly is and what the cause is, in order to provide the best treatment possible for the patient. -

The Lacrimal System Terms
The Lacrimal System Lynn E. Lawrence, CPOT, ABOC, COA, OSC Terms • Etiology – the cause of a disease or abnormal condition • Dacryocystitis – inflammation of the lacrimal sac • Epiphora – watering of eyes due to excess secretion of tears or obstruction of the lacrimal passage Tear Film Layers oil aqueous snot What functions does each layer of the tear perform? Lacrimal System: Tear Film Layers LIPID DEFICIENCY ‐ evaporates TEAR DEFICIENCY – fails to hydrate properly oil aqueous snot What functions does each layer of the tear perform? What are functions of tears? Tear Components • Lipid Layer – prevents evaporation • Aqueous Layer ‐ hydration • Mucus Layer – sticks tear to the eye • Other components Lacrimal Apparatus • Sometimes a person cannot produce natural tears they might need punctal plugs to prevent the tears from draining off the eye. • Faucet • Action • Drain Obstructive – vs‐ non‐obstructive Tear Production – Secretory • Lacrimal gland – Reflex tearing – Too much tearing…epiphora • Gland of Krause – Superior fornix • Gland of Wolfring – Superior tarsal plate Two Primary Forms of Dry Eye 800 nm 8,000 nm 100 nm The two primary forms of dry eye are Evaporative Dry Eye, also known as Meibomian Gland Dysfunction or MGD and Aqueous Dry Eye. The majority of dry eye sufferers have MGD. Oil & Water Remember science class? Oil floats. Oil does not mix with water, but rather sits on top of water. Oil is what keeps water from evaporating. Need three volunteers TEST TIME http://optometrytimes.modernmedicine.com/optometrytimes/news/treating‐dry‐eye‐ lipid‐based‐eye‐drops Lipid Secretion: Meibomian Glands Left: Transillumination of eyelid showing meibomian glands Right: Secretion of lipid at lid margin • The lipid layer restricts evaporation to 5‐10% of tear flow – Also helps lubricate Mucin Secretion: Goblet Cells Superficial layer of bulbar conjunctiva. -

Surgical Management of Carcinoma Ofeyelids and Periorbital Skin
Br J Ophthalmol: first published as 10.1136/bjo.63.8.578 on 1 August 1979. Downloaded from British Journal of Ophthalmology, 1979, 63, 578-585 Surgical management of carcinoma of eyelids and periorbital skin HEMANT MEHTA From the Department of Ophthalmology, Caernarvonshire and Anglesey General Hospital, Bangor, Gwynedd SUMMARY An appraisal of a personal series of 115 unselected and surgically treated cutaneous cancers of palpebral region is presented. Histological confirmation of the diagnosis and adequacy of excision was obtained for all lesions. Seven of the 8 patients with doubtful clearance were successfully treated with further surgery very soon. Complications were few, the incidence of re- operations low, and cosmetic as well as functional results were mostly satisfactory. Tumour recurred in 1 case (087%). Two patients had a poor cosmetic result. Seventy-nine cases (69%) were treated as day cases under local anaesthesia even for major repairs like full-thickness reconstruction of two-thirds of the lower eyelid and repairs with large full-thickness skin grafts of up to 20 x 55 mm by a new simple technique of graft fixation. The use of longer-acting local anaesthetics in oculoplastic surgery is described. Attention is drawn to the dangers of using direct wound closure for repair. Apart from the readily observable cosmetic blemish 2 by radiotherapy and 4 by surgery, including a that they produce malignant tumours of the skin rodent ulcer that was curetted elsewhere, having of the eyelids and periorbita differ from cutaneous been mistaken for a meibomian cyst. All excised malignancies elsewhere by their tendency to damage lesions were examined histologically for confirma- the ocular and adnexal structures either by direct tion of diagnosis and completeness of excision. -
Abducens Nerve Lesion • Right LR Is Disabled
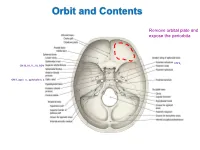
Orbit and Contents Remove orbital plate and expose the periorbita CN V2 CN III, IV, V1, VI, SOV CN II, optic n., ophthalmic a. Orbit and Contents Contents of orbit: • Eyeball and extraocular mm • Nerves to mm and orbital contents • Optic nerve (CN II) – retina; vision • Ciliary ganglion – PS supply to sphincter pupillae and ciliary mm • Ophthalmic artery and veins • Lacrimal gland and ducts • Lacrimal sac and canaliculi • FAT!! Bony Orbit and Walls The orbit is a 4-sided pyramid: Base (orbital margin) – anterolaterally • Frontal, Maxilla, Zygomatic bones Apex - posteromedially Walls: Roof – frontal bone Lateral – zygomatic, greater wing of sphenoid Medial – lesser wing of sphenoid, ethmoid (lamina papyracea), lacrimal, part of maxilla Floor – maxilla (“roof of maxillary sinus”) Three of the four sides are bounded by paranasal sinuses: Superiorly – Frontal sinus (behind eyebrows) Medially – Ethmoidal sinuses (air cells) Inferiorly – Maxillary sinus Bones are thin and vulnerable to “blowout” fractures Bony Orbit and Openings Orbital and Optical Axes and Position of Eyeball in Orbit = visual axis Axes about which movements of the eyeball occur 1. Vertical axis (superior/inferior poles) – rotation of pupil toward nose (=adduction) – rotation of pupil away from nose (=abduction) 2. Transverse axis (mediolateral; at equator) – rotate pupil upward (=elevation) – rotate pupil downward (=depression) 3. Anterior-posterior axis (optical axis, line of sight) – rotate superior pole medially (=intorsion) – rotate superior pole laterally (=extorsion) Only the medial rectus and lateral rectus have pure, singular actions. All others move the eyeball on 3 different axes. Extraocular Muscles Muscle Origin Insertion Innervation Function Levator palpebrae Lesser wing of sphenoid Skin of upper eyelid Oculomotor n. -

The Special Senses Objectives • Describe the Sensory Organs of Smell, and Olfaction
The Special Senses Objectives • Describe the sensory organs of smell, and olfaction. • Identify the accessory and internal structures of the eye, and explain their function. • Explain how light stimulates the production of nerve impulses and vision. • Describe the structures of the external and middle ear and explain how they function. • Describe the parts of the inner ear and their roles in equilibrium and hearing. Olfactory organs • Contain olfactory epithelium with olfactory receptors, supporting cells, basal cells • Olfactory receptors are modified neurons • Olfaction detects dissolved chemicals as they interact with odorant binding proteins Olfaction • Olfactory pathways • No synapse in the thalamus for arriving information • Olfactory discrimination • Can distinguish thousands of chemical stimuli • CNS interprets smells by pattern of receptor activity • Olfactory receptor population shows considerable turnover • Number of receptors declines with age Gustation Taste receptors • Clustered in taste buds • Associated with lingual papillae Taste buds • Gustatory cells extend taste hairs through a narrow taste pore Gustatory pathways • Taste buds are monitored by cranial nerves • Synapse with the medulla oblongata, then thalamus and the primary sensory cortex Gustatory discrimination • Primary taste sensations • Sweet, sour, salty, bitter • Receptors also exist for umami and water • Taste sensitivity shows significant individual differences, some of which are inherited • The number of taste buds declines with age 82 Vision Accessory structures -

Jemds.Com Original Research Article
Jemds.com Original Research Article ANALYSIS OF 34 CASES OF ENDONASAL ENDOSCOPIC DACRYOCYSTORHINOSTOMY- SURGICAL SUCCESS AND PATIENT SATISFACTION, A CASE SERIES, OUR EXPERIENCE N. Gopinathan Pillai1, Binu Babu2, Anjana Mary Reynolds3, Subadhra S4 1Associate Professor, Department of Otorhinolaryngology, PIMSRC, Thiruvalla. 2Assistant Professor, Department of Otorhinolaryngology, PIMSRC, Thiruvalla. 3Assistant Professor, Department of Otorhinolaryngology, PIMSRC, Thiruvalla. 4Junior Resident, Department of Otorhinolaryngology, PIMSRC, Thiruvalla. ABSTRACT BACKGROUND The conventional treatment of dacryocystitis is external dacryocystorhinostomy. Its success rate varies from 80 - 98%.1-4 But patient’s satisfaction was poor due to facial scar, disruption of medial canthus anatomy and dysfunction of lacrimal pump mechanism. Endoscopic DCR has neither facial scar nor any postoperative distortion of lacrimal pump mechanism and medial canthal anatomy. The objective of this study is to assess the surgical success rate and patient’s satisfaction after endonasal endoscopic Dacryocystorhinostomy (DCR). Study Design- This study was done at Pushpagiri Institute of Medical Sciences and Research Centre, Thiruvalla, between January 2012 and August 2016. There were 34 patients included in this study. Females are more commonly affected than males. Unilateral cases are more than bilateral cases. Their age ranges from 13 - 83 years. Mean age is 35 years. MATERIALS AND METHODS Patients presented with epiphora or swelling below the medial canthus of eye with or without pain, mucopurulent regurgitation from the lacrimal sac into the eye on pressing the swelling. Five patients had concomitant deviated nasal septum, for which septoplasty was done along with DCR. The patency of nasolacrimal duct was assessed by syringing and diagnostic nasal endoscopy. RESULTS The success rate is comparable to other studies of endonasal DCR. -

ORGAN of VISION the Organ of Vision Includes: 1) Eyeball 2
ORGAN OF VISION The organ of vision includes: 1) Eyeball 2) Auxiliary apparatus (eyebrows, palpebrae, conjunctiva, lacrimal apparatus, extrinsic eye muscles, fat tissue of an orbit), 3) Optic nerve and visual pathway. EYEBALL The eyeball is located in the orbit. It is surrounded by a fibrous sheath (Tenon’s capsule). Between the capsule and the walls of an orbit adipose tissue is located, between the capsule and the eyeball – episcleral (Tenon’s) spase. Eyeball has two poles: anterior and posterior. The line connecting these poles outside – external axis of the eye (an average of about 24 mm), inside – internal axis of the eye (about 22 mm). Thus, external axis connects the convex part of the external surface of the cornea to the convex part of the external surface of the sclera. Internal axis connects the convex part of the internal surface of the cornea to the convex part of the internal surface of the sclera. The line connecting the anterior pole (the convex part of the cornea) with the point of the best vision (center of yellow spot of the retina) - optic axis. Equator of the eye divides it into anterior and posterior segments. The eyeball consists of the nucleus and tunics (layers). LAYERS OF THE EYEBALL: 1. Fibrous tunic 2. Vascular tunic 3. Internal tunic 1.FIBROUS TUNIC It is divided on two parts: the anterior – cornea, posterior – sclera. Cornea is transparent. It hasn’t blood vessels, its shape is convex and thus it refracts light rays coming into the eye. Posterior large part is a sclera (sclerous or albuminous shell = «white»).