A Randomized Controlled Trial of the Effect of Supervised Progressive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

P1697 Abstract (Poster Session) Characterisation of Carbapenem Non-Susceptible Pseudomonas Aeruginosa Isolates in Danish Hospitals; a Nationwide Study F
P1697 Abstract (poster session) Characterisation of carbapenem non-susceptible Pseudomonas aeruginosa isolates in Danish hospitals; a nationwide study F. Hansen*, U.S. Justesen, C. Østergaard, H.K. Johansen, M. Arpi, D.S. Hansen, P. Littauer, A. Holm, O. Heltberg, H. Schumacher, K. Fuursted, M.-A. Lykke, B. Tønning, A.M. Hammerum (Copenhagen S, Odense, Aalborg, Copenhagen, Herlev, Hillerød, Hvidovre, Vejle, Slagelse, Herning, Aarhus, Esbjerg, Viborg, DK) Objectives: In many European countries an increase in carbapenem non-susceptible Pseudomonas aerugionosa has been observed. Until 2011, no systematic data from Denmark had been registered, so a consecutive collection of carbapenem resistant P. aeruginosa was enacted, to investigate the carbapenem resistance mechanisms in Danish P. aeruginosa isolates. Methods: From January 1st 2011 through June 30th 2011, 116 nonreplicate, non-cystic fibrosis related P. aeruginosa isolates with reduced carbapenem susceptibility were collected from 12 out of 13 Danish Departments of Clinical Microbiology. The presence of acquired beta- lactamases was assessed using a combination tablet method, and the isolates were antimicrobial susceptibility tested against relevant antipseudomonal agents. Beta-lactamase subgroup specific PCR assays, subsequent sequencing analysis as well as an efflux pump inhibitor assay were performed. Results: Eight isolates produced the metallo-betalactamase VIM-2 and one isolate produced both OXA-10 and a VEB-group enzyme. Furthermore, 67 isolates displayed a derepressed AmpC phenotype, deduced from cloxacillin or boronic acid synergy with either ceftazidime or meropenem. Phenotypic indications of increased efflux pump activity were seen in 44 isolates. Efflux and AmpC positive results occurred more frequently in isolates resistant to both meropenem and imipenem than in isolates resistant only to imipenem. -

Increased Incidence of Mycoplasma Pneumoniae Infections Detected by Laboratory-Based Surveillance in Denmark in 2010
Rapid communications Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010 J N Rasmussen1, M Voldstedlund1, R L Andersen2, S Ellermann-Eriksen3, T G Jensen4, H K Johansen5, B Kolmos6, M Mølvadgaard7, S S Nielsen1, E Olsen8, K Schønning9, S A Uldum ([email protected])1 1. Statens Serum Institut, Copenhagen, Denmark 2. Clinical Microbiology Department, Nordsjællands Hospital, Hillerød, Denmark 3. Clinical Microbiology Department, Århus University Hospital, Skejby, Denmark 4. Clinical Microbiology Department, Odense University Hospital, Odense, Denmark 5. Clinical Microbiology Department, Rigshospitalet, Copenhagen, Denmark 6. Clinical Microbiology Department, Sygehus Lillebælt, Vejle, Denmark 7. Clinical Microbiology Department, Aalborg Sygehus Syd, Aalborg, Denmark 8. Clinical Microbiology Department, Regionshospitalet Viborg, Skive,Viborg, Denmark 9. Clinical Microbiology Department, Hvidovre Hospital, Hvidovre, Denmark Citation style for this article: Rasmussen JN, Voldstedlund M, Andersen RL, Ellermann-Eriksen S, Jensen TG, Johansen HK, Kolmos B, Mølvadgaard M, Nielsen SS, Olsen E, Schønning K, Uldum SA. Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Euro Surveill. 2010;15(45):pii=19708. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19708 Article published on 11 November 2010 In Denmark recurrent epidemics of Mycoplasma pneu- the local clinical microbiology departments have moniae infections have been described since the taken over a large part of the laboratory tests for 1950s at intervals of approximately four to six years. M. pneumoniae. The diagnosis had previously been The latest epidemic occurred in 2004/05 followed by based on serology but since the beginning of the 1990s two years of high incidence and more than three years PCR has been introduced as a routine diagnostic test of low incidence. -

Theme: Recent Advances in MR Technology – Ready for Clinical Use?
Joint MR meeting between ISMRM & SMRT Nordic Chapter and DSMMR October 22-23, 2019 Rigshospitalet, Copenhagen Theme: Recent advances in MR technology – ready for clinical use? Tuesday - 22.10.2019 11.00 Registration Session 1 - Oncology - MRI in combination with other modalities Chairs: Tone Frost Bathen, Norwegian Univ. of Science and Technology (NTNU), Trondheim, NO; Dora Grauballe, Århus University Hospital, Århus, DEN 12.00 Welcome - Hartwig Siebner , Danish Research Centre for Magnetic Resonance (DRCMR), Copenhagen University Hospital Hvidovre, Hvidovre, DEN 12.05 Five poster Pitches 12.15 Vendor presentation Clinical Scientist and MR physicist Karen Kettless, Siemens Healthineers 12.25 Whole-body MRI in oncology - clinical studies (Speaker: Erik Morre Pedersen, Århus University Hospital, Århus, DEN ) 12.50 Applications of PET-MRI in the management of brain tumors (Speaker: Live Eikenes, Dept. of Circulation & Medical Imaging, NTNU, Trondheim, NOR ) 13.15 Hybrid PET/MR in clinical brain imaging - opportunities, challenges and experiences. (Speaker: Otto M. Henriksen. MD, PhD, Consultant, Section of PET, Dept. of Clin. Physiology, Nuclear Medicine & PET, Rigshopitalet, Copenhagen, DEN ) 13.40 MR-linac – Potential and challenges for radiation oncology (Speaker: Faisal Mahmood, Odense University Hospital, Odense, DEN) 14.05 Free presentation (selected from abstracts) 14.15 Free presentation (selected from abstracts) 14.25 Break Session 2 - Progress in molecular imaging – ready for clinical use in patients? Chair: Karin Markenroth Bloch, -
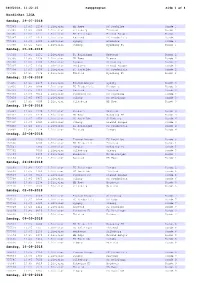
Kampprogram Side 1 Af 4
08062018, 11:22:25 Kampprogram Side 1 af 4 NordicBet LIGA Søndag, 29-07-2018 755383 13:45 1238 1.Division HB Køge FC Roskilde Runde 1 755385 13:45 7000 1.Division Silkeborg Thisted Runde 1 755386 13:45 1231 1.Division FC Helsingør Fremad Amager Runde 1 755387 13:45 1414 1.Division Næstved FC Fredericia Runde 1 755389 13:45 1380 1.Division Lyngby Hvidovre Runde 1 755390 13:45 6465 1.Division Viborg Nykøbing FC Runde 1 Søndag, 05-08-2018 755391 13:45 1231 1.Division FC Helsingør Næstved Runde 2 755395 13:45 1238 1.Division HB Køge Viborg Runde 2 755396 13:45 1380 1.Division Lyngby Silkeborg Runde 2 755397 13:45 2130 1.Division Hvidovre Fremad Amager Runde 2 755398 13:45 1456 1.Division FC Roskilde FC Fredericia Runde 2 755399 13:45 7774 1.Division Thisted Nykøbing FC Runde 2 Søndag, 12-08-2018 755435 13:45 2169 1.Division Fremad Amager Lyngby Runde 3 755436 13:45 6088 1.Division FC Fredericia Hvidovre Runde 3 755437 13:45 1414 1.Division Næstved Thisted Runde 3 755438 13:45 9510 1.Division Nykøbing FC FC Roskilde Runde 3 755439 13:45 6465 1.Division Viborg FC Helsingør Runde 3 755440 13:45 7000 1.Division Silkeborg HB Køge Runde 3 Søndag, 19-08-2018 755441 13:45 2130 1.Division Hvidovre Næstved Runde 4 755443 13:45 1238 1.Division HB Køge Nykøbing FC Runde 4 755494 13:45 1456 1.Division FC Roskilde Silkeborg Runde 4 755495 13:45 6465 1.Division Viborg Fremad Amager Runde 4 755496 13:45 1231 1.Division FC Helsingør FC Fredericia Runde 4 755497 13:45 7774 1.Division Thisted Lyngby Runde 4 Onsdag, 22-08-2018 755499 2169 1.Division Fremad Amager -
Study Protocol
Protocol for generic testing of antigen test for SARS-CoV-2 in Denmark Prepared and relevantly modified according to STARD 2015 and the SPIRIT 2013 statement for reporting of diagnostic accuracy studies and standard protocol items for clinical trials. Administrative information Title Agreement of antigen tests on oral pharyngeal swabs or less invasive testing with RT-PCR, for detecting SARS-CoV-2 in adults: A prospective nationwide observational study Trial registration www.clinicaltrials.gov Protocol version May 19th, 2021; v2.4 Funding Companies providing antigen tests, hereafter termed the providers. - The provider delivers 800 antigen-tests free-of-charge. - The provider will pay a divided share of the cost of performing the additional testing of individuals suspected or diagnosed with SARS-CoV-2 and the RT-PCR retesting of previously positive individuals. - The provider will pay a divided share of the costs of the administrative, analytical work and open access publishing. - All funds are collected at a trust account administrated by Hvidovre Hospital, Region Hovedstaden and cost for regional testing is covered from this account. Any excess funds will be divided between the providers when the study is published. The planned RT-PCR testing for SARS-CoV-2 of individuals with suspected COVID-19 infection is conducted as planned by the Danish health-care system Roles and The study will be open as a national project welcoming all Departments of Clinical responsibilities Microbiology (DCM) in Denmark, all test centers and all medical companies that wishes to promote antigen tests in Denmark. The steering group will: write and publish the protocol, assess which tests that have the documented quality to be included in the study, oversee the execution of the study, data analysis and drafting of manuscript. -

Mænd Masters 30-34
Mænd masters 30-34 år: 100m 11.58 Anders Sækmose (74) 22.05.07 Aarhus 200m 24.1 Holger Bagger (43) 14.09.76 Aarhus 400m 53.5 Benno Jensen (45) 14.06.79 Vejle 800m 1.57.2 Benno Jensen (45) 24.07.75 Lyngby 1000m 2.33.6 Benno Jensen (45) 16.07.75 Lyngby 1500m 4.01.0 Benno Jensen (45) 17.06.75 Aarhus 1 mile 4.34.1 Allan Kehlet (46) 07.07.76 Odense 2000m 5.36.0 Benno Jensen (45) 02.07.75 Lyngby 3000m 8.49.3 John Jacobsen (63) 01.09.93 Vejle 5000m 15.10.99 John Jacobsen (63) 18.09.93 Skovdalen 10000m 32.20.5 Svend Erik Pedersen (51) 08.05.84 Aarhus 1 dansk mil 23.54 John Jacobsen (63) 24.01.93 Aarhus 10km landevej 31.46 John Jacobsen (63) 12.12.93 Viborg 15km 48.49 John Jacobsen (63) 04.04.93 Viborg 20km 1.07.26.4 Allan Kehlet (46) 22.01.78 Aarhus 25km 1.24.08 John Jacobsen (63) 19.11.94 San Diego, USA 30km 1.58.09 Bruno Ejlskov (63) 28.08.93 Randers ½-marathon 1.17.15 Jan M. Iversen (61) 22.10.95 Vejle marathon 2.30.31 John Jacobsen (63) 22.01.95 San Diego, USA 100km 11.10.07.0 Chr. Panbo (41) 13.05.72 København kort hæk 14.5 Svend Åge Thomsen (11) 14.09.42 Vejle 110m hæk 14.66 Anders Sækmose (74) 17.06.06 Slovakiet 200m hæk 25.7 Svend Åge Thomsen (11) 16.08.42 Østerbro 400m hæk 54.7 Anders Sækmose (74) 10.06.06 Vejle 3000m forhindring 9.52.4 Svend Erik Pedersen (51) 15.06.84 Vejle højde 1,90 Tommy Jensen (58) 21.08.88 Vejle stang 4,10 Steen Andersen (42) 05.09.73 Aalborg længde 6,72 Svend Åge Thomsen (11) 16.08.42 Østerbro trespring 13,84 Erland Jørgensen (41) 28.08.71 Holstebro kugle 20,02 Ole Lindskjold (44) 14.08.74 Lyngby diskos 56,40 Peter Jarl Hansen (44) 22.05.77 Aalborg spyd 52,82 Tommy Jensen (58) 31.08.91 Vejle hammer 60,85 Ole Lindskjold (44) 11.05.74 Hvidovre vægt 19,57 Peter Jarl Hansen (44) 11.06.77 Reykjavik kaste 5-kamp 4.420 p. -

Midlertidig Oversigt 2021.Xlsx
FERIEPLAN 2021 ‐ REGION HOVEDSTADEN DERMATOLOGI GYNÆKOLOGI Uge 23 24 25 26 27 28 29 30 31 32 33 34Uge 23 24 25 26 27 28 29 30 31 32 33 34 Matthias Hans Friedrich GerberBornholm 00000UgUgUg0000 Astrid Petersen København 000000UgUgUg000 Anne Lerbæk Jørkov Frederiksberg 00000UgUgUg0000 Birgitte Marianne Rindom Ballerup 00000UgUgUgUg000 Louise Arup Neergaard Frederiksberg 00000UgUgUgUg000 Anette I. Grønning Olesen Egedal 0 Ug00000UgUg000 Marianne Anderson Frederikssund 12‐13/715/7‐ 111000 Hans Kristen Krog Frederiksberg Ferie ikke oplyst Elisabeth Ammitzbøll Furesø 000000UgUgUgUg00 Ursula Bentin‐Ley Frederiksberg Ferie ikke oplyst Ida‐Marie Stender Gentofte 0000UgUgUgUg0000 Camilla Flarup Gosvig Frederiksberg 00000UgUgUg0000 Niels Henrik Nielsen Gladsaxe 000000UgUgUgUg00 Lone Enslev Nyrnberg Frederikssund 0000UgUgUg00000 Frederik de Fine Olivarius Gladsaxe 00000UgUgUgUg000 Mette Erenbjerg Frederiksberg 000000UgUgUg000 Gitte Kiellberg Larsen Glostrup 00014/7‐‐UgUgUgUgUg 11/8 Irina Goukasian Furesø 000000UgUgUg000 Hans‐Henrik Horsten Helsingør 00000UgUgUg0000 Elisabeth Carlsen Gentofte 0000UgUgUgUgUg000 Rikke Elkjær Andersen Helsingør 00000UgUgUg0000 Kåre Rygaard Gentofte Ingen ferielukning Monika Gniadecka HerlevFerie ikke oplyst Helle Christina Sørensen Gentofte 00000UgUgUgUg000 Peter Jensen Hillerød 000UgUgUgUg00000 Anne Høyrup Bjerre Gentofte Ferie ikke oplyst Patricia Louise Danielsen HillerødFerie ikke oplyst Jacob Peter Philipsen Gladsaxe Ferie ikke oplyst Frederik Grønhøj Larsen Høje‐Taastrup 00000UgUgUgUg000 Lise Helmsøe‐Zinck Gladsaxe -

In the Event of Injury Or Sudden Illness
The Capital Region of Denmark Åben 24 timer Special uddannede lægevagt EMERGENCY MEDICALsygeplejersker SERVICES IN THE CAPITAL Åben 24 timer Special uddannede lægevagt 112 Akuttelefonen Giftlinien REGIONsygeplejersker OF DENMARK Emergency telephone 112 Your own physician (GP) lægevagt 112 Akuttelefonen ÅbenGiftlinien 24 timer SpecialEgen uddannede Læge Psykiatrisk www.regionh.dk Call 112 if you need urgent medical Yousygeplejersker can consult your own physician (GP) assistance in the event of acute life between 8 AM and 4 PM on weekdays.Your threatening illness or injury. GP can treat illnesses and minor injuries, as well as refer you to further treatment or IN THE EVENT OF Egen Læge Helpline 1813Psykiatrisk 112 Akuttelefonen GiftlinienGuide Tandlægevagt www.regionh.dk examination.God Tid Your GP´s telephone number Åben 24 timer Special uddannede lægevagt sygeplejersker Call 1813 if you need help in the event of and address are printed on your health card. INJURY OR SUDDEN Åben 24 timer Special uddannede lægevagt injury or a sudden illness. You cansygeplejersker also dial Åben 24 timer Special uddannede lægevagt Psychiatric admissions 1813sygeplejersker when you are in doubt about what ILLNESS Egen Læge Psykiatrisk www.regionh.dk God Tid Guide Tandlægevagt to do. Ambulancer og akutlægebiler Akutmodtagelser/akutklinikker 112 Akuttelefonen Giftlinien You can receive urgent help in psychiatric acute admissions/centres. Dial 1813, if The Emergency112 Centre Akuttelefonen Giftlinien you are in doubt. Psychiatric admissions 112 Akuttelefonen Giftlinien Telephone calls to 112 and 1813 are in Ballerup, Glostrup, Hvidovre, Hillerød God Tid Guide Tandlægevagt Ambulancer og akutlægebiler Akutmodtagelser/akutklinikker and Copenhagen are open 24 hours a day, Egen Læge answeredPsykiatrisk by thewww.regionh.dk regional emergency while psychiatric admissions in Amager centre, which is responsiblelægevagt for providing Åben 24 timer Special uddannede Egen Læge Psykiatrisk www.regionh.dk propersygeplejersker assistance. -

Fælleskommunale It-Samarbejder – En Oversigt
Version 4.3 af 1. maj 2020 Fælleskommunale it-samarbejder – en oversigt - Brugerklubben SBSYS - Byregion Fyn - Danskernes Digitale Bibliotek (DDB) - Den Digitale Hotline (DDH) - Den Storkøbenhavnske Digitaliseringsforening (DSD) - Det Sønderjyske Digitaliseringsnetværk - Digitaliseringsforening Sjælland (DIGIT) - Esbjerg-Varde Samarbejdet - FOSAKO - Fællesudbud Sjælland (FUS) - Fællesindkøb Fyn (FIF) - IT-Forsyningen - KOMBIT – Kommunernes it-fællesskab - KOMDA - Netværket Elektronisk Arkivering (NEA) - Netværk om leverandørstyring og -samarbejde - Nordsjællands Digitaliseringssamarbejde - Open Data DK - OS2 - Offentligt Digitaliseringsfællesskab - Sydvestjysk Samarbejde - ØS Indsigt - 3K - 5K Digital Videncenter for Digitalisering og Teknologi, www.videncenter.kl.dk, [email protected] Side 1/14 Samarbejde Formål Form Kommuner/partnere Kontakt og yderligere info Brugerklubben SBSYS Indkøbsfællesskab, hvor Forening 38 kommuner og 1 region: Jørgen Kristensen Rasch, medlemmerne i Albertslund, Ballerup, Brønderslev, [email protected] / tlf. 2159 3660 fællesskab udvikler og Faxe, Fredensborg, Frederiksberg, vedligeholder det Frederikssund, Faaborg-Midtfyn, [email protected] elektroniske sags- og Gladsaxe, Gribskov, Halsnæs, dokumenthåndterings- Hedensted, Herlev, Herning, Hjørring, www.sbsys.dk system SBSYS og Holstebro, Horsens, Ikast-Brande, tilgrænsende løsninger og Ishøj, Jammerbugt, Lolland, Lyngby- ydelser. Taarbæk, Læsø, Mariagerfjord, Næstved, Odense, Randers, Rebild, Rødovre, Skanderborg, Solrød, Sorø, Stevns, Struer, Thisted, Tønder, Vejle, -

Kvalitativ Analyse Af Udvalgte Kommuners Klimatilpasningsstrategier
Baggrundsrapport til: Klimatilpasning i de danske kommuner – et overblik Kvalitativ analyse af udvalgte kommuners klimatilpasningsstrategier ARBEJDSRAPPORT SKOV & LANDSKAB 120 / 2010 Dorthe Hedensted Lund Titel Baggrundsrapport til: Klimatilpasning i de danske kommuner – et overblik. Kvalitativ analyse af udvalgte kommuners klimatilpasnings- strategier Forfatter Dorthe Hedensted Lund Serietitel, nr. Arbejdsrapport Skov & Landskab nr. 120 Rapporten publiceres på www.sl.life.ku.dk Bedes citeret Lund, Dorthe H. (2010): Baggrundsrapport til: Klimatilpasning i de danske kommuner – et overblik. Kvalitativ analyse af udvalgte kommuners klimatilpasningsstrategier. Arbejdsrapport nr. 120, Skov & Landskab, Københavns Universitet, Frederiksberg, 45 s. ISBN 978-87-7903-514-0 Udgivere Skov & Landskab, Københavns Universitet: Nationalt center for forskning, uddannelse og rådgivning i skov og skovprodukter, landskabsarkitektur og landskabsforvaltning, byplan- lægning og bydesign Koordineringsenhed for Forskning i Klimatilpasning (KFT): er etableret under regeringens strategi for tilpasning til klimaændrin- ger i Danmark. Bag KFT står forskningsinstitutionerne: Aarhus Uni- versitet, Danmarks Meteorologiske Institut, De Nationale Geologiske Undersøgelser for Danmark og Grønland, Københavns Universitet samt Danmarks Tekniske Universitet. KFT–sekretariatet har adresse ved Danmarks Miljøundersøgelser, Aarhus Universitet KFT skal fremme tværgående videnopbygning inden for forskning i klimatilpasning samt i klima og klimaeffekter relevant for klimatilpas- ning -

Hvidovre Kommunes
FØRSTE GENERATIONS VANDPLANER 2009 - 2015 HVIDOVRE KOMMUNES VANDHANDLEPLAN HVIDOVRE KOMMUNES VANDHANDLEPLAN Dato 2015-20-03 Udarbejdet Maj til juni 2012 og revideret i januar 2015 Udarbejdet af Hvidovre Kommune Beskrivelse Vandhandleplan for Hvidovre Kommune. Vandhandleplanen implementer statens Vandplan for hovedopland 2.4 – Køge Bugt. Baggrunds Kortmaterialet er hentet fra MiljøGIS på Naturstyrelsens materiale hjemmeside i forbindelse med udarbejdelsen og opdaterin- gen af planen. OFFENTLIG HØRING Nærværende reviderede Forslag til Hvidovre Kommunes vandhandleplan har været i of- fentlig høring fra den 20. marts 2015 frem til den 15. maj 2015. VEDTAGELSE På baggrund af de indkomne indsigelser er Vandhandleplanen ….. KLAGEVEJLEDNING Hvidovres endelige Vandhandleplan kan påklages frem til den 27. november 2015 klage skal sendes til: [email protected] eller med brev til: Hvidovre Kommune Tekniske Forvaltning Høvedstensvej 45 2650 Hvidovre. Forsidefoto Kalveboderne. HVIDOVRE KOMMUNES VANDHANDLEPLAN 1. FORORD Hvorfor kommer der planer der dækker en planperiode fra 2009 – 2015? Hvidovre Kommunes vandhandleplan beskriver den kommunale udmøntning af Statens første generations vandplaner (VP1). Planperioden for VP1 strækker sig fra 2009 – 2015. De kommunale vandhandleplaner vedtages først endeligt nu på grund af for- sinkelsen af de statslige vandplaner, der danner grundlaget for Kommunernes arbejde. Statens vandplaner VP1 blev oprindeligt vedtaget i 2011, hvorefter de var i of- fentlig høring. Planen blev efterfølgende sendt i 8 dages supplerende høring. På grund af den korte supplerende høring underkendte Natur- og miljøklage- nævnet i december 2012 planen. I 2013 blev VP1 opdateret og derefter sendt i fornyet høring fra 21. juni 2013 og frem til 23. december 2013. Der efter har planen været i 8 ugers supplerende høring hen over sommeren 2014, hvor ef- ter den er rettet og godkendt 30. -

Delivery and Invoice Addresses E-Invoices
Ørsted - Delivery and invoice addresses Questions / support please contact: Ørsted - Financial Services Phone +45 9955 7000 E-mail: [email protected] e-invoices We can receive EDI by GLN/EAN in the format OIOUBL --- If you are unable to send OIOUBL, try Sproom - www.sproom.net - Free electronic invoicing --- PDF Alternative you can send invoices and credit notes in PDF to: [email protected] Only one invoice per PDF-file and papersize maximum 300dpi / A4 Statement must be send to: [email protected] Reminders must be send to: [email protected] Company Contries: Europe: America: Denmark United States Germany Netherland Asia: Poland Malaysia Sweden Taiwan United Kingdom Singapore Isle of Man Japan South Korea Updated: 13-05-2021 Ørsted - Delivery and invoice addresses Name & Delivery address Name, Invoice address & CVR / Company reg. no. & Ørsted GLN/EAN no. VAT no. CC: Denmark Ørsted A/S Ørsted A/S CVR DK36213728 1000 Kraftværksvej 53, Skærbæk Kraftværksvej 53 VAT no. DK36213728 DK-7000 Fredericia DK-7000 Fredericia Ørsted A/S GLN/EAN: 5790001793067 Nesa Allé 1 DK-2820 Gentofte Ørsted Services A/S Ørsted Services A/S CVR DK27446485 9000 Kraftværksvej 53 Kraftværksvej 53 VAT no. DK36213728 DK-7000 Fredericia DK-7000 Fredericia Ørsted Services A/S GLN/EAN: 5790001793081 Nesa Allé 1 DK-2820 Gentofte Ørsted Wind Power A/S Ørsted Wind Power A/S CVR DK31849292 7600 Kraftværksvej 53 Kraftværksvej 53 Danish DK36213728 VAT no. DK-7000 Fredericia DK-7000 Fredericia English GB894510014 VAT no. German DE290739647 VAT no. GLN/EAN: 5790001793678 Dutch NL823996037B01 VAT no. Belgian BE0561888831 VAT no. French FR44799320007 VAT no.