Characteristics and Outcomes After Confirmed Infection in Pregnancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kommunenavn: Svendborg Kommuners Indberetning På
Kommunenavn: Svendborg Kommuners indberetning på godsområdet til beregning af økonomisk kompensationsbehov som følge af COVID-19 Billetindtægter er ekskl. ordinært § 21 a tilskud, vareafgift til havnene samt moms Beregning af mindreindtægter Billetindtægter i Billetindtægter i perioden marts- perioden marts- september i 2019 (i september i 2020 (i Kommune Rute 1.000 kr.) 1.000 kr.) Assens Baagø-Assens Fanø Fanø-Esbjerg Faaborg-Midtfyn Bjørnø-Faaborg Faaborg-Midtfyn Lyø-Avernakø-Faaborg Haderslev Aarø-Aarøsund Hedensted Hjarnø-Snaptun Holbæk Orø-Holbæk Holbæk Orø- Hammer bakker Horsens Endelave-Snaptun Kalundborg Havnsø-Sejerø Kalundborg Havnsø-Nekselø Langeland Strynø-Rudkøbing Lolland Fejø-Kragenæs Lolland Femø-Kragenæs Lolland Askø-Bandholm Læsø Læsø-Frederikshavn Norddjurs Anholt-Grenaa Odder Tunø-Hou Samsø Hou-Samsø Skive Fur-Branden Slagelse Agersø-Stigsnæs Slagelse Omø-Stigsnæs Struer Venø-Kleppen Svendborg Hjortø-Svendborg 1 3 Svendborg Skarø-drejø-Svendborg 139 111 Ærø Birkholm-Marstal Ærø Ærøskøbing-Svendborg Ærø Søby-Faaborg Ærø Søby-Fynshav Ærø Marstal-Rudkøbing Aabenraa Barsø-Barsø-Landing Aalborg Egholm-Aalborg Kommuners indberetning på godsområdet til beregning af økonomisk kompensationsbehov som følge af COVID-19 Beregning af mindreindtægter Samlede mindreindtægte r i 2020 (i 1.000 kr.) 2 -28 Kommunenavn: Svendborg Kommuners indberetning på passagerområdet til beregning af økonomisk kompensationsbehov som følge af COVID-19 Billetindtægt er ekskl. ordinært § 21 b tilskud og moms Beregning af mindreindtægter Billetindtægter -

P1697 Abstract (Poster Session) Characterisation of Carbapenem Non-Susceptible Pseudomonas Aeruginosa Isolates in Danish Hospitals; a Nationwide Study F
P1697 Abstract (poster session) Characterisation of carbapenem non-susceptible Pseudomonas aeruginosa isolates in Danish hospitals; a nationwide study F. Hansen*, U.S. Justesen, C. Østergaard, H.K. Johansen, M. Arpi, D.S. Hansen, P. Littauer, A. Holm, O. Heltberg, H. Schumacher, K. Fuursted, M.-A. Lykke, B. Tønning, A.M. Hammerum (Copenhagen S, Odense, Aalborg, Copenhagen, Herlev, Hillerød, Hvidovre, Vejle, Slagelse, Herning, Aarhus, Esbjerg, Viborg, DK) Objectives: In many European countries an increase in carbapenem non-susceptible Pseudomonas aerugionosa has been observed. Until 2011, no systematic data from Denmark had been registered, so a consecutive collection of carbapenem resistant P. aeruginosa was enacted, to investigate the carbapenem resistance mechanisms in Danish P. aeruginosa isolates. Methods: From January 1st 2011 through June 30th 2011, 116 nonreplicate, non-cystic fibrosis related P. aeruginosa isolates with reduced carbapenem susceptibility were collected from 12 out of 13 Danish Departments of Clinical Microbiology. The presence of acquired beta- lactamases was assessed using a combination tablet method, and the isolates were antimicrobial susceptibility tested against relevant antipseudomonal agents. Beta-lactamase subgroup specific PCR assays, subsequent sequencing analysis as well as an efflux pump inhibitor assay were performed. Results: Eight isolates produced the metallo-betalactamase VIM-2 and one isolate produced both OXA-10 and a VEB-group enzyme. Furthermore, 67 isolates displayed a derepressed AmpC phenotype, deduced from cloxacillin or boronic acid synergy with either ceftazidime or meropenem. Phenotypic indications of increased efflux pump activity were seen in 44 isolates. Efflux and AmpC positive results occurred more frequently in isolates resistant to both meropenem and imipenem than in isolates resistant only to imipenem. -

Apparat Firma Analysegruppe Analyseinterface Slagelse Vejle
Apparat Firma Analysegruppe Analyseinterface Interface Slagelse Vejle Odense Sønderborg Esbjerg Rigshospitalet Thorshavn Aarhus Bactec Becton Dickinson (BD) bloddyrkning BACTEC Ja Ja Ja Ja Nej Bact-Alert Biomerieux bloddyrkning BACTALERT ja Ja Ja Veriti 96 Thermal cycler * 2 Ab Applied Biosystem/ Life Technoglies – Thermo Fisher Scientific PCR nej Mx3005P - Stratagene AH-diagnostic - Agilent PCR nej nej nej NucliSENS easyMAG Biomerieux PCR nej Viper Becton Dickinson (BD) PCR ja BEP2000 Siemens Elisa BEP2000 (ja) (Ja) Nej Liaison XL DiaSorin Antistofpåvisning LIAISON ja nej Ja Ja Ja???? Ja Ja LIAISON_COV Manuel indlæsning af data fra Novo el. DTU Multiopsætning LIAISON_COV_AUTO Automatisk indlæsning af data fra Novo el. DTU Multiopsætning Maldi Tof Bruker Daltonics Scandinavia AB Bakt. identifikation MALDI ja Ja Ja nej Ja Ja VitekMS Biomerieux Bakt. identifikation EPI_CENTER Ja Ja Ja Galaxy matrixpip.system Bruker Daltonics Scandinavia AB Afpippetering Nej WASP SSI Diagnostica Udsåningsrobot Ja Nej Ja Nej Nej Kiestra Becton Dickinson (BD) Udsåningsrobot Ja BD MAX Becton Dickinson (BD) PCR BDMAX Ja Nej GeneXpert Diagen PCR GENEXPERT ja Ja Ja Nej Nej Nej Sofia SOFIA ja Ja Bullet Pro Triolab/ DiaSorin PCR Nej QIAxcel Advanced System Qiagen PCR Nej Nej AriaMx (Agilent), AH-diagnostic PCR Nej Arrow Triolab/ DiaSorin PCR Nej Panther Hologic TMA PANTHER Ja Ja Ja Tigris Hologic TMA TIGRIS Ja FilmArray (BioFire/biomerieux) FILMARRAY Nej Nej Nej Nej Stratagene Mx3005 Agilent Nej QuantStudio (Thermofischer scientific) Nej Roche(KBA) Roche Nej Taqman -

Increased Incidence of Mycoplasma Pneumoniae Infections Detected by Laboratory-Based Surveillance in Denmark in 2010
Rapid communications Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010 J N Rasmussen1, M Voldstedlund1, R L Andersen2, S Ellermann-Eriksen3, T G Jensen4, H K Johansen5, B Kolmos6, M Mølvadgaard7, S S Nielsen1, E Olsen8, K Schønning9, S A Uldum ([email protected])1 1. Statens Serum Institut, Copenhagen, Denmark 2. Clinical Microbiology Department, Nordsjællands Hospital, Hillerød, Denmark 3. Clinical Microbiology Department, Århus University Hospital, Skejby, Denmark 4. Clinical Microbiology Department, Odense University Hospital, Odense, Denmark 5. Clinical Microbiology Department, Rigshospitalet, Copenhagen, Denmark 6. Clinical Microbiology Department, Sygehus Lillebælt, Vejle, Denmark 7. Clinical Microbiology Department, Aalborg Sygehus Syd, Aalborg, Denmark 8. Clinical Microbiology Department, Regionshospitalet Viborg, Skive,Viborg, Denmark 9. Clinical Microbiology Department, Hvidovre Hospital, Hvidovre, Denmark Citation style for this article: Rasmussen JN, Voldstedlund M, Andersen RL, Ellermann-Eriksen S, Jensen TG, Johansen HK, Kolmos B, Mølvadgaard M, Nielsen SS, Olsen E, Schønning K, Uldum SA. Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Euro Surveill. 2010;15(45):pii=19708. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19708 Article published on 11 November 2010 In Denmark recurrent epidemics of Mycoplasma pneu- the local clinical microbiology departments have moniae infections have been described since the taken over a large part of the laboratory tests for 1950s at intervals of approximately four to six years. M. pneumoniae. The diagnosis had previously been The latest epidemic occurred in 2004/05 followed by based on serology but since the beginning of the 1990s two years of high incidence and more than three years PCR has been introduced as a routine diagnostic test of low incidence. -

An Immigrant Story of Peter Petersen Thisted: Itinerant, Maverik Danish Lutheran Pastor, 1859-1915
The Bridge Volume 17 Number 1 Article 7 1994 An Immigrant Story Of Peter Petersen Thisted: Itinerant, Maverik Danish Lutheran Pastor, 1859-1915 Paul A. Thisted Follow this and additional works at: https://scholarsarchive.byu.edu/thebridge Part of the European History Commons, European Languages and Societies Commons, and the Regional Sociology Commons Recommended Citation Thisted, Paul A. (1994) "An Immigrant Story Of Peter Petersen Thisted: Itinerant, Maverik Danish Lutheran Pastor, 1859-1915," The Bridge: Vol. 17 : No. 1 , Article 7. Available at: https://scholarsarchive.byu.edu/thebridge/vol17/iss1/7 This Article is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in The Bridge by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. An Immigrant Story of Peter Petersen Thisted: Itinerant, Maverick Danish Lutheran Pastor, 1859-1915 "Without struggle, no victory, without victory, no crown." 2 Tim. 2:5 by PAUL A. THISTED Introduction This is the story of my Danish grandfather whom I never knew except through infrequent stories from my father. I asked my father Aaron to write a family history about his dad, but he never did. I never found out why he wouldn't. I wondered about my Thisted grandparents before and after both my parents died, so I decided to piece the puzzle together as best I could, while several uncles and aunts were still alive. My best resource was my sister, LaJune Barth, who had saved my grandfather's sermons, books, almanacs, and miscellaneous papers after the death of our mother, who had saved them for six years after Dad had died. -

Coastal Living in Denmark
To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Coastal living in Denmark © Daniel Overbeck - VisitNordsjælland To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. The land of endless beaches In Denmark, we look for a touch of magic in the ordinary, and we know that travel is more than ticking sights off a list. It’s about finding wonder in the things you see and the places you go. One of the wonders that we at VisitDenmark are particularly proud of is our nature. Denmark has wonderful beaches open to everyone, and nowhere in the nation are you ever more than 50km from the coast. s. 2 © Jill Christina Hansen To change the color of the coloured box, right-click here and select Format Background, change the color as shown in the picture on the right. Denmark and its regions Geography Travel distances Aalborg • The smallest of the Scandinavian • Copenhagen to Odense: Bornholm countries Under 2 hours by car • The southernmost of the • Odense to Aarhus: Under 2 Scandinavian countries hours by car • Only has a physical border with • Aarhus to Aalborg: Under 2 Germany hours by car • Denmark’s regions are: North, Mid, Jutland West and South Jutland, Funen, Aarhus Zealand, and North Zealand and Copenhagen Billund Facts Copenhagen • Video Introduction • Denmark’s currency is the Danish Kroner Odense • Tipping is not required Zealand • Most Danes speak fluent English Funen • Denmark is of the happiest countries in the world and Copenhagen is one of the world’s most liveable cities • Denmark is home of ‘Hygge’, New Nordic Cuisine, and LEGO® • Denmark is easily combined with other Nordic countries • Denmark is a safe country • Denmark is perfect for all types of travelers (family, romantic, nature, bicyclist dream, history/Vikings/Royalty) • Denmark has a population of 5.7 million people s. -

Juleture 2020 Fyn - Sjælland Afg Mod Sjælland Ca
Juleture 2020 Fyn - Sjælland Afg mod Sjælland ca. kl. 10:00 Svendborg Odense Nyborg ↔ København Afg mod fyn ca. kl.15:00 kr. 150 Afg mod Sjælland ca. kl. 10:00 Svendborg Odense Nyborg ↔ Roskilde Hillerød Helsingør Afg mod fyn ca. kl.15:00 kr. 150 Afg mod Sjælland ca. kl. 10:00 Svendborg Odense Nyborg ↔ Næstved Vordingborg Nykøbing F Afg mod fyn ca. kl.15:00 kr. 150 Afg mod Sjælland ca. kl. 10:00 Svendborg Odense Nyborg ↔ Korsør Slagelse Sorø Ringsted Køge Afg mod fyn ca. kl.15:00 kr. 150 Jylland - Fyn Afg mod Fyn ca. kl. 10:00 Aalborg Hobro Randers ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 200 Afg mod Fyn ca. kl. 10:00 Aarhus ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 150 Afg mod Fyn ca. kl. 10:00 Viborg Silkeborg ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 150 Afg mod Fyn ca. kl. 10:00 Holstebro Herning ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 150 Afg mod Fyn ca. kl. 10:00 Esbjerg Kolding Fredericia ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 150 Afg mod Fyn ca. kl. 10:00 Skanderborg Horsens Vejle ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 150 Afg mod Fyn ca. kl. 10:00 Sønderborg Aabenraa Haderslev ↔ Middelfart Odense Svendborg Afg mod Jylland ca. kl.15:00 kr. 150 Jylland - Sjælland Afg mod Sjælland ca. kl. 10:00 Aalborg Hobro Randers ↔ København Afg mod Jylland ca. -

Private-Public Innovation Project
GRUNDFOS BIOBOOSTER PRIVATE-PUBLIC INNOVATION PROJECT WASTEWATER TREATMENT AT HERLEV HOSPITAL, DENMARK BACKGROUND AND PROJECT OBJECTIVES HOSPITAL WASTEWATER Herlev Hospital, located in the However, (BAT) the best available Project deliverables also include: are needed to improve the removal hazardous substances and patho- CONTAINS A COMPLEX Capital Region of Denmark, faces techniques (here specifically tech- - demonstrating that air emissions of pollutants. The test programme gens. Therefore risk assessments MIXTURE OF HAZARDOUS these wastewater challenges as nology that is both technically and can be treated for pathogens and includes the evaluation of robust- are crucial instruments to evaluate PHARMACEUTICALS, the Danish government wants to economically feasible to treat the odour. ness, energy consumption and total whether the treatment technolo- HARMFUL PATHOGENS ensure that hospital wastewater hospital wastewater) has not yet - demonstrating that the by-prod- economic performance under long- gies can remove all the important AND ANTIBIOTIC is handled according to the Envi- been defined and therefore the re- ucts can be disposed of in an en- term operating conditions. risks whilst at the same time not RESISTENT BACTERIA. ronmental Protection Act’s regu- quirements for any discharge per- vironmentally sound manner. create any unintended risks. DHI lations concerning the use of the mit cannot be specified at present. - Developing a control method to will carry out environmental and THE TEST PROGRAMME THESE CANNOT BE RE- Best Available Techniques (BAT). detect when GAC filters are satu- health risk assessments in relation MOVED BY CONVENTIONAL INCLUDES THE EVALUATION Their objectives are to seek a treat- The purpose of this project is to ad- rated in order to optimise replace- OF ROBUSTNESS, ENERGY to all emissions from the WWTP MEANS OF WASTEWATER ment solution for Herlev Hospi- dress the above objectives and has ment intervals. -

Theme: Recent Advances in MR Technology – Ready for Clinical Use?
Joint MR meeting between ISMRM & SMRT Nordic Chapter and DSMMR October 22-23, 2019 Rigshospitalet, Copenhagen Theme: Recent advances in MR technology – ready for clinical use? Tuesday - 22.10.2019 11.00 Registration Session 1 - Oncology - MRI in combination with other modalities Chairs: Tone Frost Bathen, Norwegian Univ. of Science and Technology (NTNU), Trondheim, NO; Dora Grauballe, Århus University Hospital, Århus, DEN 12.00 Welcome - Hartwig Siebner , Danish Research Centre for Magnetic Resonance (DRCMR), Copenhagen University Hospital Hvidovre, Hvidovre, DEN 12.05 Five poster Pitches 12.15 Vendor presentation Clinical Scientist and MR physicist Karen Kettless, Siemens Healthineers 12.25 Whole-body MRI in oncology - clinical studies (Speaker: Erik Morre Pedersen, Århus University Hospital, Århus, DEN ) 12.50 Applications of PET-MRI in the management of brain tumors (Speaker: Live Eikenes, Dept. of Circulation & Medical Imaging, NTNU, Trondheim, NOR ) 13.15 Hybrid PET/MR in clinical brain imaging - opportunities, challenges and experiences. (Speaker: Otto M. Henriksen. MD, PhD, Consultant, Section of PET, Dept. of Clin. Physiology, Nuclear Medicine & PET, Rigshopitalet, Copenhagen, DEN ) 13.40 MR-linac – Potential and challenges for radiation oncology (Speaker: Faisal Mahmood, Odense University Hospital, Odense, DEN) 14.05 Free presentation (selected from abstracts) 14.15 Free presentation (selected from abstracts) 14.25 Break Session 2 - Progress in molecular imaging – ready for clinical use in patients? Chair: Karin Markenroth Bloch, -

The Status, Characteristics and Potential of SMART SPECIALISATION in Nordic Regions
The status, characteristics and potential of SMART SPECIALISATION in Nordic Regions By Mari Wøien, Iryna Kristensen and Jukka Teräs NORDREGIO REPORT 2019:3 nordregio report 2019:3 1 The status, characteristics and potential of SMART SPECIALISATION in Nordic Regions By Mari Wøien, Iryna Kristensen and Jukka Teräs NORDREGIO REPORT 2019:3 Prepared on behalf of the Nordic Thematic Group for Innovative and Resilient Regions 2017–2020, under the Nordic Council of Ministers Committee of Civil Servants for Regional Affairs. The status, characteristics and potential of smart specialisation in Nordic Regions Nordregio Report 2019:3 ISBN 978-91-87295-67-6 ISSN 1403-2503 DOI: doi.org/10.30689/R2019:3.1403-2503 © Nordregio 2019 Nordregio P.O. Box 1658 SE-111 86 Stockholm, Sweden [email protected] www.nordregio.org www.norden.org Analyses and text: Mari Wøien, Iryna Kristensen and Jukka Teräs Contributors: Ágúst Bogason, Eeva Turunen, Laura Fagerlund, Tuulia Rinne and Viktor Salenius, Nordregio. Cover: Taneli Lahtinen Nordregio is a leading Nordic and European research centre for regional development and planning, established by the Nordic Council of Ministers in 1997. We conduct solution-oriented and applied research, addressing current issues from both a research perspective and the viewpoint of policymakers and practitioners. Operating at the international, national, regional and local levels, Nordregio’s research covers a wide geographic scope, with an emphasis on the Nordic and Baltic Sea Regions, Europe and the Arctic. The Nordic co-operation Nordic co-operation is one of the world’s most extensive forms of regional collaboration, involving Denmark, Finland, Iceland, Norway, Sweden, and the Faroe Islands, Greenland, and Åland. -
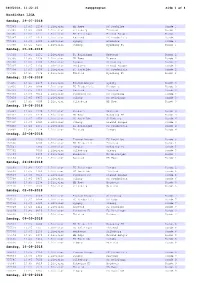
Kampprogram Side 1 Af 4
08062018, 11:22:25 Kampprogram Side 1 af 4 NordicBet LIGA Søndag, 29-07-2018 755383 13:45 1238 1.Division HB Køge FC Roskilde Runde 1 755385 13:45 7000 1.Division Silkeborg Thisted Runde 1 755386 13:45 1231 1.Division FC Helsingør Fremad Amager Runde 1 755387 13:45 1414 1.Division Næstved FC Fredericia Runde 1 755389 13:45 1380 1.Division Lyngby Hvidovre Runde 1 755390 13:45 6465 1.Division Viborg Nykøbing FC Runde 1 Søndag, 05-08-2018 755391 13:45 1231 1.Division FC Helsingør Næstved Runde 2 755395 13:45 1238 1.Division HB Køge Viborg Runde 2 755396 13:45 1380 1.Division Lyngby Silkeborg Runde 2 755397 13:45 2130 1.Division Hvidovre Fremad Amager Runde 2 755398 13:45 1456 1.Division FC Roskilde FC Fredericia Runde 2 755399 13:45 7774 1.Division Thisted Nykøbing FC Runde 2 Søndag, 12-08-2018 755435 13:45 2169 1.Division Fremad Amager Lyngby Runde 3 755436 13:45 6088 1.Division FC Fredericia Hvidovre Runde 3 755437 13:45 1414 1.Division Næstved Thisted Runde 3 755438 13:45 9510 1.Division Nykøbing FC FC Roskilde Runde 3 755439 13:45 6465 1.Division Viborg FC Helsingør Runde 3 755440 13:45 7000 1.Division Silkeborg HB Køge Runde 3 Søndag, 19-08-2018 755441 13:45 2130 1.Division Hvidovre Næstved Runde 4 755443 13:45 1238 1.Division HB Køge Nykøbing FC Runde 4 755494 13:45 1456 1.Division FC Roskilde Silkeborg Runde 4 755495 13:45 6465 1.Division Viborg Fremad Amager Runde 4 755496 13:45 1231 1.Division FC Helsingør FC Fredericia Runde 4 755497 13:45 7774 1.Division Thisted Lyngby Runde 4 Onsdag, 22-08-2018 755499 2169 1.Division Fremad Amager -

Page 1 P E R N I L L E
P E R N I L L E P O N T O P P I D A N P E D E R S E N Born in 1987 in Skive, Denmark Lives and work on a farm in the woods around Silkeborg, Denmark Pernille Pontoppidan Pedersen started her career as a ceramist by undermining the bases of this discipline. She developed an aesthetic of the ugly, the failure, the missed attempt. In one of her first series of work, she chose to disregard her training to excellence by integrating to her art the failed experiences of the best ceramic artists of the country. She Kills the jar, the pot, bowl and all the craft objects, to which ceramics limited itself for a long time. She undermines her discipline, and starts to reconstruct: shaky and almighty structures, tiered tempetto- pieces, sandcastles, heaps of found objects caught in a heavy flow of clay, foamy glazes that overflow like washing up fluid. The works of Pernille Pontoppidan tell a hundred pieces of fascinated stories superimposed in a cadaver exquis of good and very bad taste, of baroque and minimalism, of extreme dexterity and bitter failures. E D U C A T I O N 2012 BA in ceramics, Royal Danish Academy, School of Architecture, Design and Conservation, the school of design, Bornholm, Denmark E X H I B I T I O N S 2019 Chart Art fair, with Køppe Contemporary, Copenhagen Ceramic Momentum, group exhibition, CLAY Museum, Copenhagen 2018 Chart Art fair, with Køppe Contemporary, Copenhagen Dialogue through time, group exhibition, Wilds’sches Haus, Basel 2017 Beyond Icons, group exhibition, Koldinghus TRESOR Art Fair, with Køppe Contemporary,