Study Protocol and Statistical Analysis Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

P1697 Abstract (Poster Session) Characterisation of Carbapenem Non-Susceptible Pseudomonas Aeruginosa Isolates in Danish Hospitals; a Nationwide Study F
P1697 Abstract (poster session) Characterisation of carbapenem non-susceptible Pseudomonas aeruginosa isolates in Danish hospitals; a nationwide study F. Hansen*, U.S. Justesen, C. Østergaard, H.K. Johansen, M. Arpi, D.S. Hansen, P. Littauer, A. Holm, O. Heltberg, H. Schumacher, K. Fuursted, M.-A. Lykke, B. Tønning, A.M. Hammerum (Copenhagen S, Odense, Aalborg, Copenhagen, Herlev, Hillerød, Hvidovre, Vejle, Slagelse, Herning, Aarhus, Esbjerg, Viborg, DK) Objectives: In many European countries an increase in carbapenem non-susceptible Pseudomonas aerugionosa has been observed. Until 2011, no systematic data from Denmark had been registered, so a consecutive collection of carbapenem resistant P. aeruginosa was enacted, to investigate the carbapenem resistance mechanisms in Danish P. aeruginosa isolates. Methods: From January 1st 2011 through June 30th 2011, 116 nonreplicate, non-cystic fibrosis related P. aeruginosa isolates with reduced carbapenem susceptibility were collected from 12 out of 13 Danish Departments of Clinical Microbiology. The presence of acquired beta- lactamases was assessed using a combination tablet method, and the isolates were antimicrobial susceptibility tested against relevant antipseudomonal agents. Beta-lactamase subgroup specific PCR assays, subsequent sequencing analysis as well as an efflux pump inhibitor assay were performed. Results: Eight isolates produced the metallo-betalactamase VIM-2 and one isolate produced both OXA-10 and a VEB-group enzyme. Furthermore, 67 isolates displayed a derepressed AmpC phenotype, deduced from cloxacillin or boronic acid synergy with either ceftazidime or meropenem. Phenotypic indications of increased efflux pump activity were seen in 44 isolates. Efflux and AmpC positive results occurred more frequently in isolates resistant to both meropenem and imipenem than in isolates resistant only to imipenem. -

Increased Incidence of Mycoplasma Pneumoniae Infections Detected by Laboratory-Based Surveillance in Denmark in 2010
Rapid communications Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010 J N Rasmussen1, M Voldstedlund1, R L Andersen2, S Ellermann-Eriksen3, T G Jensen4, H K Johansen5, B Kolmos6, M Mølvadgaard7, S S Nielsen1, E Olsen8, K Schønning9, S A Uldum ([email protected])1 1. Statens Serum Institut, Copenhagen, Denmark 2. Clinical Microbiology Department, Nordsjællands Hospital, Hillerød, Denmark 3. Clinical Microbiology Department, Århus University Hospital, Skejby, Denmark 4. Clinical Microbiology Department, Odense University Hospital, Odense, Denmark 5. Clinical Microbiology Department, Rigshospitalet, Copenhagen, Denmark 6. Clinical Microbiology Department, Sygehus Lillebælt, Vejle, Denmark 7. Clinical Microbiology Department, Aalborg Sygehus Syd, Aalborg, Denmark 8. Clinical Microbiology Department, Regionshospitalet Viborg, Skive,Viborg, Denmark 9. Clinical Microbiology Department, Hvidovre Hospital, Hvidovre, Denmark Citation style for this article: Rasmussen JN, Voldstedlund M, Andersen RL, Ellermann-Eriksen S, Jensen TG, Johansen HK, Kolmos B, Mølvadgaard M, Nielsen SS, Olsen E, Schønning K, Uldum SA. Increased incidence of Mycoplasma pneumoniae infections detected by laboratory-based surveillance in Denmark in 2010. Euro Surveill. 2010;15(45):pii=19708. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19708 Article published on 11 November 2010 In Denmark recurrent epidemics of Mycoplasma pneu- the local clinical microbiology departments have moniae infections have been described since the taken over a large part of the laboratory tests for 1950s at intervals of approximately four to six years. M. pneumoniae. The diagnosis had previously been The latest epidemic occurred in 2004/05 followed by based on serology but since the beginning of the 1990s two years of high incidence and more than three years PCR has been introduced as a routine diagnostic test of low incidence. -

The Double-Sided Effects of Mycobacterium Bovis Bacillus Calmette–Guérin Vaccine ✉ ✉ Junli Li 1,2,3,4, Lingjun Zhan1,2,3,4 and Chuan Qin 1,2,3,4
www.nature.com/npjvaccines REVIEW ARTICLE OPEN The double-sided effects of Mycobacterium Bovis bacillus Calmette–Guérin vaccine ✉ ✉ Junli Li 1,2,3,4, Lingjun Zhan1,2,3,4 and Chuan Qin 1,2,3,4 Bacillus Calmette–Guérin (BCG), the only vaccine proven to be effective against tuberculosis (TB), is the most commonly used vaccine globally. In addition to its effects on mycobacterial diseases, an increasing amount of epidemiological and experimental evidence accumulated since its introduction in 1921 has shown that BCG also exerts non-specific effects against a number of diseases, such as non-mycobacterial infections, allergies and certain malignancies. Recent Corona Virus Disease 2019 (COVID-19) outbreak has put BCG, a classic vaccine with significant non-specific protection, into the spotlight again. This literature review briefly covers the diverse facets of BCG vaccine, providing new perspectives in terms of specific and non-specific protection mechanisms of this old, multifaceted, and controversial vaccine. npj Vaccines (2021) 6:14 ; https://doi.org/10.1038/s41541-020-00278-0 INTRODUCTION infections observed in children after receiving BCG vaccina- 17–20 Bacillus Calmette–Guérin (BCG), a live-attenuated bacterial tion . Clinical evidence also suggests that BCG may be 1234567890():,; effective against infections caused by viral pathogens, such as vaccine derived from Mycobacterium bovis was originally – 1 respiratory syncytial virus21,22, human papilloma virus23 25,and isolated in 1902 from a cow with tuberculosis (TB) .Theisolate 26 was cultured continuously for >230 generations for 13 years herpes simplex virus . Moreover, an increasing number of (1908–1921) to generate a mutant strain with weakened animal studies using mouse models have demonstrated the virulence but with high immunogenicity2. -

Theme: Recent Advances in MR Technology – Ready for Clinical Use?
Joint MR meeting between ISMRM & SMRT Nordic Chapter and DSMMR October 22-23, 2019 Rigshospitalet, Copenhagen Theme: Recent advances in MR technology – ready for clinical use? Tuesday - 22.10.2019 11.00 Registration Session 1 - Oncology - MRI in combination with other modalities Chairs: Tone Frost Bathen, Norwegian Univ. of Science and Technology (NTNU), Trondheim, NO; Dora Grauballe, Århus University Hospital, Århus, DEN 12.00 Welcome - Hartwig Siebner , Danish Research Centre for Magnetic Resonance (DRCMR), Copenhagen University Hospital Hvidovre, Hvidovre, DEN 12.05 Five poster Pitches 12.15 Vendor presentation Clinical Scientist and MR physicist Karen Kettless, Siemens Healthineers 12.25 Whole-body MRI in oncology - clinical studies (Speaker: Erik Morre Pedersen, Århus University Hospital, Århus, DEN ) 12.50 Applications of PET-MRI in the management of brain tumors (Speaker: Live Eikenes, Dept. of Circulation & Medical Imaging, NTNU, Trondheim, NOR ) 13.15 Hybrid PET/MR in clinical brain imaging - opportunities, challenges and experiences. (Speaker: Otto M. Henriksen. MD, PhD, Consultant, Section of PET, Dept. of Clin. Physiology, Nuclear Medicine & PET, Rigshopitalet, Copenhagen, DEN ) 13.40 MR-linac – Potential and challenges for radiation oncology (Speaker: Faisal Mahmood, Odense University Hospital, Odense, DEN) 14.05 Free presentation (selected from abstracts) 14.15 Free presentation (selected from abstracts) 14.25 Break Session 2 - Progress in molecular imaging – ready for clinical use in patients? Chair: Karin Markenroth Bloch, -
Kampprogram Side 1 Af 4
08062018, 11:22:25 Kampprogram Side 1 af 4 NordicBet LIGA Søndag, 29-07-2018 755383 13:45 1238 1.Division HB Køge FC Roskilde Runde 1 755385 13:45 7000 1.Division Silkeborg Thisted Runde 1 755386 13:45 1231 1.Division FC Helsingør Fremad Amager Runde 1 755387 13:45 1414 1.Division Næstved FC Fredericia Runde 1 755389 13:45 1380 1.Division Lyngby Hvidovre Runde 1 755390 13:45 6465 1.Division Viborg Nykøbing FC Runde 1 Søndag, 05-08-2018 755391 13:45 1231 1.Division FC Helsingør Næstved Runde 2 755395 13:45 1238 1.Division HB Køge Viborg Runde 2 755396 13:45 1380 1.Division Lyngby Silkeborg Runde 2 755397 13:45 2130 1.Division Hvidovre Fremad Amager Runde 2 755398 13:45 1456 1.Division FC Roskilde FC Fredericia Runde 2 755399 13:45 7774 1.Division Thisted Nykøbing FC Runde 2 Søndag, 12-08-2018 755435 13:45 2169 1.Division Fremad Amager Lyngby Runde 3 755436 13:45 6088 1.Division FC Fredericia Hvidovre Runde 3 755437 13:45 1414 1.Division Næstved Thisted Runde 3 755438 13:45 9510 1.Division Nykøbing FC FC Roskilde Runde 3 755439 13:45 6465 1.Division Viborg FC Helsingør Runde 3 755440 13:45 7000 1.Division Silkeborg HB Køge Runde 3 Søndag, 19-08-2018 755441 13:45 2130 1.Division Hvidovre Næstved Runde 4 755443 13:45 1238 1.Division HB Køge Nykøbing FC Runde 4 755494 13:45 1456 1.Division FC Roskilde Silkeborg Runde 4 755495 13:45 6465 1.Division Viborg Fremad Amager Runde 4 755496 13:45 1231 1.Division FC Helsingør FC Fredericia Runde 4 755497 13:45 7774 1.Division Thisted Lyngby Runde 4 Onsdag, 22-08-2018 755499 2169 1.Division Fremad Amager -
Study Protocol
Protocol for generic testing of antigen test for SARS-CoV-2 in Denmark Prepared and relevantly modified according to STARD 2015 and the SPIRIT 2013 statement for reporting of diagnostic accuracy studies and standard protocol items for clinical trials. Administrative information Title Agreement of antigen tests on oral pharyngeal swabs or less invasive testing with RT-PCR, for detecting SARS-CoV-2 in adults: A prospective nationwide observational study Trial registration www.clinicaltrials.gov Protocol version May 19th, 2021; v2.4 Funding Companies providing antigen tests, hereafter termed the providers. - The provider delivers 800 antigen-tests free-of-charge. - The provider will pay a divided share of the cost of performing the additional testing of individuals suspected or diagnosed with SARS-CoV-2 and the RT-PCR retesting of previously positive individuals. - The provider will pay a divided share of the costs of the administrative, analytical work and open access publishing. - All funds are collected at a trust account administrated by Hvidovre Hospital, Region Hovedstaden and cost for regional testing is covered from this account. Any excess funds will be divided between the providers when the study is published. The planned RT-PCR testing for SARS-CoV-2 of individuals with suspected COVID-19 infection is conducted as planned by the Danish health-care system Roles and The study will be open as a national project welcoming all Departments of Clinical responsibilities Microbiology (DCM) in Denmark, all test centers and all medical companies that wishes to promote antigen tests in Denmark. The steering group will: write and publish the protocol, assess which tests that have the documented quality to be included in the study, oversee the execution of the study, data analysis and drafting of manuscript. -

BCG Coverage and Barriers to BCG Vaccination In
Thysen et al. BMC Public Health 2014, 14:1037 http://www.biomedcentral.com/1471-2458/14/1037 RESEARCH ARTICLE Open Access BCG coverage and barriers to BCG vaccination in Guinea-Bissau: an observational study Sanne Marie Thysen1,2*, Stine Byberg1,2, Marie Pedersen1,2, Amabelia Rodrigues1, Henrik Ravn2,3, Cesario Martins1, Christine Stabell Benn2,3, Peter Aaby1,2,3 and Ane Bærent Fisker1,2 Abstract Background: BCG vaccination is recommended at birth in low-income countries, but vaccination is often delayed. Often 20-dose vials of BCG are not opened unless at least ten children are present for vaccination (“restricted vial-opening policy”). BCG coverage is usually reported as 12-month coverage, not disclosing the delay in vaccination. Several studies show that BCG at birth lowers neonatal mortality. We assessed BCG coverage at different ages and explored reasons for delay in BCG vaccination in rural Guinea-Bissau. Methods: Bandim Health Project (BHP) runs a health and demographic surveillance system covering women and their children in 182 randomly selected village clusters in rural Guinea-Bissau. BCG coverage was assessed for children born in 2010, when the restricted vial-opening policy was universally implemented, and in 2012–2013, where BHP provided BCG to all children at monthly visits in selected intervention regions. Factors associated with delayed BCG vaccination were evaluated using logistic regression models. Coverage between intervention and control regions were evaluated in log-binomial regression models providing prevalence ratios. Results: Among 3951 children born in 2010, vaccination status was assessed for 84%. BCG coverage by 1 week of age was 11%, 38% by 1 month, and 92% by 12 months. -

Mænd Masters 30-34
Mænd masters 30-34 år: 100m 11.58 Anders Sækmose (74) 22.05.07 Aarhus 200m 24.1 Holger Bagger (43) 14.09.76 Aarhus 400m 53.5 Benno Jensen (45) 14.06.79 Vejle 800m 1.57.2 Benno Jensen (45) 24.07.75 Lyngby 1000m 2.33.6 Benno Jensen (45) 16.07.75 Lyngby 1500m 4.01.0 Benno Jensen (45) 17.06.75 Aarhus 1 mile 4.34.1 Allan Kehlet (46) 07.07.76 Odense 2000m 5.36.0 Benno Jensen (45) 02.07.75 Lyngby 3000m 8.49.3 John Jacobsen (63) 01.09.93 Vejle 5000m 15.10.99 John Jacobsen (63) 18.09.93 Skovdalen 10000m 32.20.5 Svend Erik Pedersen (51) 08.05.84 Aarhus 1 dansk mil 23.54 John Jacobsen (63) 24.01.93 Aarhus 10km landevej 31.46 John Jacobsen (63) 12.12.93 Viborg 15km 48.49 John Jacobsen (63) 04.04.93 Viborg 20km 1.07.26.4 Allan Kehlet (46) 22.01.78 Aarhus 25km 1.24.08 John Jacobsen (63) 19.11.94 San Diego, USA 30km 1.58.09 Bruno Ejlskov (63) 28.08.93 Randers ½-marathon 1.17.15 Jan M. Iversen (61) 22.10.95 Vejle marathon 2.30.31 John Jacobsen (63) 22.01.95 San Diego, USA 100km 11.10.07.0 Chr. Panbo (41) 13.05.72 København kort hæk 14.5 Svend Åge Thomsen (11) 14.09.42 Vejle 110m hæk 14.66 Anders Sækmose (74) 17.06.06 Slovakiet 200m hæk 25.7 Svend Åge Thomsen (11) 16.08.42 Østerbro 400m hæk 54.7 Anders Sækmose (74) 10.06.06 Vejle 3000m forhindring 9.52.4 Svend Erik Pedersen (51) 15.06.84 Vejle højde 1,90 Tommy Jensen (58) 21.08.88 Vejle stang 4,10 Steen Andersen (42) 05.09.73 Aalborg længde 6,72 Svend Åge Thomsen (11) 16.08.42 Østerbro trespring 13,84 Erland Jørgensen (41) 28.08.71 Holstebro kugle 20,02 Ole Lindskjold (44) 14.08.74 Lyngby diskos 56,40 Peter Jarl Hansen (44) 22.05.77 Aalborg spyd 52,82 Tommy Jensen (58) 31.08.91 Vejle hammer 60,85 Ole Lindskjold (44) 11.05.74 Hvidovre vægt 19,57 Peter Jarl Hansen (44) 11.06.77 Reykjavik kaste 5-kamp 4.420 p. -

Midlertidig Oversigt 2021.Xlsx
FERIEPLAN 2021 ‐ REGION HOVEDSTADEN DERMATOLOGI GYNÆKOLOGI Uge 23 24 25 26 27 28 29 30 31 32 33 34Uge 23 24 25 26 27 28 29 30 31 32 33 34 Matthias Hans Friedrich GerberBornholm 00000UgUgUg0000 Astrid Petersen København 000000UgUgUg000 Anne Lerbæk Jørkov Frederiksberg 00000UgUgUg0000 Birgitte Marianne Rindom Ballerup 00000UgUgUgUg000 Louise Arup Neergaard Frederiksberg 00000UgUgUgUg000 Anette I. Grønning Olesen Egedal 0 Ug00000UgUg000 Marianne Anderson Frederikssund 12‐13/715/7‐ 111000 Hans Kristen Krog Frederiksberg Ferie ikke oplyst Elisabeth Ammitzbøll Furesø 000000UgUgUgUg00 Ursula Bentin‐Ley Frederiksberg Ferie ikke oplyst Ida‐Marie Stender Gentofte 0000UgUgUgUg0000 Camilla Flarup Gosvig Frederiksberg 00000UgUgUg0000 Niels Henrik Nielsen Gladsaxe 000000UgUgUgUg00 Lone Enslev Nyrnberg Frederikssund 0000UgUgUg00000 Frederik de Fine Olivarius Gladsaxe 00000UgUgUgUg000 Mette Erenbjerg Frederiksberg 000000UgUgUg000 Gitte Kiellberg Larsen Glostrup 00014/7‐‐UgUgUgUgUg 11/8 Irina Goukasian Furesø 000000UgUgUg000 Hans‐Henrik Horsten Helsingør 00000UgUgUg0000 Elisabeth Carlsen Gentofte 0000UgUgUgUgUg000 Rikke Elkjær Andersen Helsingør 00000UgUgUg0000 Kåre Rygaard Gentofte Ingen ferielukning Monika Gniadecka HerlevFerie ikke oplyst Helle Christina Sørensen Gentofte 00000UgUgUgUg000 Peter Jensen Hillerød 000UgUgUgUg00000 Anne Høyrup Bjerre Gentofte Ferie ikke oplyst Patricia Louise Danielsen HillerødFerie ikke oplyst Jacob Peter Philipsen Gladsaxe Ferie ikke oplyst Frederik Grønhøj Larsen Høje‐Taastrup 00000UgUgUgUg000 Lise Helmsøe‐Zinck Gladsaxe -

EPI-NEWS NATIONAL SURVEILLANCE of COMMUNICABLE DISEASES Editor: Peter Henrik Andersen Dept
EPI-NEWS NATIONAL SURVEILLANCE OF COMMUNICABLE DISEASES Editor: Peter Henrik Andersen Dept. of Epidemiology Tel.: +45 3268 3268 • Fax: +45 3268 3874 Statens Serum Institut • 5 Artillerivej • DK 2300 Copenhagen S www.ssi.dk • [email protected] • ISSN: 1396-4798 DANISH HEALTH RESEARCH IN GUINEA-BISSAU No. 34, 2006 In one of the world’s poorest years, the BHP has been the focus of yields 70% protection against countries, Guinea-Bissau in West 22 PhD theses, mainly Danish, and rotavirus diarrhoea, and 52% Africa, the Bandim Health Project nine doctoral dissertations. protection against reinfection during (BHP) has been engaged in epide- the first year following the initial miological research since 1978 Research focus areas infection. This high protection rate focussing on infection, vaccination Two-dose measles vaccination suggests that a rotavirus vaccine and the long-term effects of health strategy: would be an effective means to intervention. Infection with measles before the reduce the incidence of acute The BHP is a collaboration between WHO recommended vaccination age diarrhoea with dehydration and Statens Serum Institut and the at 9 months is a growing problem in associated deaths. Guinea-Bissau Ministry of Health. the developing countries. This is For further information on the BHP, Child mortality in Guinea-Bissau due, among others, to increasing please contact project secretary ranks among the highest worldwide: urbanisation and an increase in the Christina Rasmussen, [email protected]. > 200 per 1000 live born infants share of mothers who were them- (P. Valentiner-Branth, K. Mølbak, during the first three years of life. selves measles vaccinated at a young Dept. -

In the Event of Injury Or Sudden Illness
The Capital Region of Denmark Åben 24 timer Special uddannede lægevagt EMERGENCY MEDICALsygeplejersker SERVICES IN THE CAPITAL Åben 24 timer Special uddannede lægevagt 112 Akuttelefonen Giftlinien REGIONsygeplejersker OF DENMARK Emergency telephone 112 Your own physician (GP) lægevagt 112 Akuttelefonen ÅbenGiftlinien 24 timer SpecialEgen uddannede Læge Psykiatrisk www.regionh.dk Call 112 if you need urgent medical Yousygeplejersker can consult your own physician (GP) assistance in the event of acute life between 8 AM and 4 PM on weekdays.Your threatening illness or injury. GP can treat illnesses and minor injuries, as well as refer you to further treatment or IN THE EVENT OF Egen Læge Helpline 1813Psykiatrisk 112 Akuttelefonen GiftlinienGuide Tandlægevagt www.regionh.dk examination.God Tid Your GP´s telephone number Åben 24 timer Special uddannede lægevagt sygeplejersker Call 1813 if you need help in the event of and address are printed on your health card. INJURY OR SUDDEN Åben 24 timer Special uddannede lægevagt injury or a sudden illness. You cansygeplejersker also dial Åben 24 timer Special uddannede lægevagt Psychiatric admissions 1813sygeplejersker when you are in doubt about what ILLNESS Egen Læge Psykiatrisk www.regionh.dk God Tid Guide Tandlægevagt to do. Ambulancer og akutlægebiler Akutmodtagelser/akutklinikker 112 Akuttelefonen Giftlinien You can receive urgent help in psychiatric acute admissions/centres. Dial 1813, if The Emergency112 Centre Akuttelefonen Giftlinien you are in doubt. Psychiatric admissions 112 Akuttelefonen Giftlinien Telephone calls to 112 and 1813 are in Ballerup, Glostrup, Hvidovre, Hillerød God Tid Guide Tandlægevagt Ambulancer og akutlægebiler Akutmodtagelser/akutklinikker and Copenhagen are open 24 hours a day, Egen Læge answeredPsykiatrisk by thewww.regionh.dk regional emergency while psychiatric admissions in Amager centre, which is responsiblelægevagt for providing Åben 24 timer Special uddannede Egen Læge Psykiatrisk www.regionh.dk propersygeplejersker assistance. -
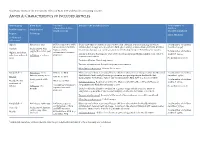
Annex A: Characteristics of Included Articles
Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines ANNEX A: CHARACTERISTICS OF INCLUDED ARTICLES Article group Birth dates Vaccines Details of the included article Total number of administered (type, children Ref ID (citation) Study period strain, reason) described/analysed Region Follow up Effect Modifier (Additional references)1 Algeria Birth dates: 1935 BCG vaccinated vs. BCG Quasi-randomised controlled trial conducted in a Muslim population in Algeria where Total number of children unvaccinated (Institute children born in 1935 were allocated to BCG (given orally) or unvaccinated at birth. Children described: 41,307 #9283(1) Study period: May Pasteur, orally revaccinated at ages 1, 3, 7 and 15 years were followed up for up to 6 months after vaccine. 1935 to December 1947 Total number of children Algiers, periurban, administered, research Inclusion Criteria: Participants were newborns from Algerian Muslim families with very low analysed: 39,259 suburban and rural Follow up: ≤ 12 years purpose) socioeconomic status. areas No modifier reported Exclusion Criteria: Not clearly stated Vaccine ascertainment: Recorded at point of vaccination Mortality ascertainment: Routine home visits Bangladesh A Birth dates: born BCG vs. no BCG Observational cohort reporting all-cause child mortality from 70 villages within the Maternal Total number of children before 1 January 2000 Child Health And Family Planning programme area participating in the Health And described: 39,625 #797(2) DPT vs. no DPT Demographic Surveillance