Used As Reference Item (Analytical Standard)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter the Influence of Nutrients and Non-CO2 Greenhouse Gas Emissions on the Ecological Footprint of Products
Quantifying effects of physical, chemical and biological stressors in life cycle assessment Hanafiah, M.M (2013). Quantifying the effects of physical, chemical and biological stressors in life cycle assessment. PhD thesis, Radboud University Nijmegen, the Netherlands. © 2013 Marlia Mohd Hanafiah, all rights reserved. Layout: Samsul Fathan Mashor & Marlia Mohd Hanafiah Printed by: S&T Photocopy Center, Bangi Quantifying the effects of physical, chemical and biological stressors in life cycle assessment Proefschrift ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann volgens besluit van het college van decanen in het openbaar te verdedigen op dinsdag 2 April 2013 om 15:30 uur precies door Marlia Mohd Hanafiah geboren op 5 September 1980 te Negeri Sembilan, Maleisië Promotoren Prof. dr. ir. A.J. Hendriks Prof. dr. M.A.J. Huijbregts Co-promotor Dr. R.S.E.W. Leuven Manuscriptcommissie Prof. dr. A.M. Breure Prof. dr. A.J.M. Smits Prof. dr. H.C. Moll (Rijksuniversiteit Groningen) Paranimfen Samsul Fathan Mashor Anastasia Fedorenkova Aan Samsul, Sophia en Sarra Table of Contents Chapter 1 Introduction 7 Chapter 2 The influence of nutrients and non-CO2 23 greenhouse gas emissions on the ecological footprint of products Chapter 3 Comparing the ecological footprint with the 48 biodiversity footprint of products Chapter 4 Characterization factors for thermal pollution 69 in freshwater aquatic environments Chapter 5 Characterization factors for water consumption -

Why Do We Have So Many Different Hydrological Models?
Why do we have so many different hydrological models? A review based on the case of Switzerland Pascal Horton*1, Bettina Schaefli1, and Martina Kauzlaric1 1Institute of Geography & Oeschger Centre for Climate Change Research, University of Bern, Bern, Switzerland ([email protected]) This is a preprint of a manuscript submitted to WIREs Water. 1 Abstract Hydrology plays a central role in applied as well as fundamental environmental sciences, but it is well known to suffer from an overwhelming diversity of models, in particular to simulate streamflow. Based on Switzerland's example, we discuss here in detail how such diversity did arise even at the scale of such a small country. The case study's relevance stems from the fact that Switzerland shows a relatively high density of academic and research institutes active in the field of hydrology, which led to an evolution of hydrological models that stands exemplarily for the diversification that arose at a larger scale. Our analysis summarizes the main driving forces behind this evolution, discusses drawbacks and advantages of model diversity and depicts possible future evolutions. Although convenience seems to be the main driver so far, we see potential change in the future with the advent of facilitated collaboration through open sourcing and code sharing platforms. We anticipate that this review, in particular, helps researchers from other fields to understand better why hydrologists have so many different models. 1 Introduction Hydrological models are essential tools for hydrologists, be it for operational flood forecasting, water resource management or the assessment of land use and climate change impacts. -

Download (9MB)
Beiträge zur Hydrologie der Schweiz Nr. 39 Herausgegeben von der Schweizerischen Gesellschaft für Hydrologie und Limnologie (SGHL) und der Schweizerischen Hydrologischen Kommission (CHy) Daniel Viviroli und Rolf Weingartner Prozessbasierte Hochwasserabschätzung für mesoskalige Einzugsgebiete Grundlagen und Interpretationshilfe zum Verfahren PREVAH-regHQ | downloaded: 23.9.2021 Bern, Juni 2012 https://doi.org/10.48350/39262 source: Hintergrund Dieser Bericht fasst die Ergebnisse des Projektes „Ein prozessorientiertes Modellsystem zur Ermitt- lung seltener Hochwasserabflüsse für beliebige Einzugsgebiete der Schweiz – Grundlagenbereit- stellung für die Hochwasserabschätzung“ zusammen, welches im Auftrag des Bundesamtes für Um- welt (BAFU) am Geographischen Institut der Universität Bern (GIUB) ausgearbeitet wurde. Das Pro- jekt wurde auf Seiten des BAFU von Prof. Dr. Manfred Spreafico und Dr. Dominique Bérod begleitet. Für die Bereitstellung umfangreicher Messdaten danken wir dem BAFU, den zuständigen Ämtern der Kantone sowie dem Bundesamt für Meteorologie und Klimatologie (MeteoSchweiz). Daten Die im Bericht beschriebenen Daten und Resultate können unter der folgenden Adresse bezogen werden: http://www.hydrologie.unibe.ch/projekte/PREVAHregHQ.html. Weitere Informationen erhält man bei [email protected]. Druck Publikation Digital AG Bezug des Bandes Hydrologische Kommission (CHy) der Akademie der Naturwissenschaften Schweiz (scnat) c/o Geographisches Institut der Universität Bern Hallerstrasse 12, 3012 Bern http://chy.scnatweb.ch Zitiervorschlag -

Gographie Illustre De La Suisse L'usage Des Coles Et Des Familles;
LIBRARY OF CONGRESS. -7^^ @]^t..V.... inîojrig^ :|n Shelf-_-.W_'^... UNITED STATES OF AMERICA. i i k mmmw umm DE LA SUISSE À l'usage DES ÉCOLES ET DES FAMILLES M. WASER PROFESSEUR À l'ÉCOLE NORMALE DE SCHWYTZ TRADUCTION FRANÇAISE PAR LE mxmim scmeuwly DIRECTEUR DES ECOLES A FRIBOURG ,3^ y il/ il i , . EINSIEDELN, NEW-YORK, CINCINNATI & ST. LOUIS CHAELES & NICOLAS BENZIGER FRERES ÉDITEURS- IMPRIMEURS 1882. Copyright 1882 by Benziger Brothers. „AU rights reserued." ^%% ^ PRÉFACE DU TMDIICTEIR. feur la demande de M. M. Benziger, libraires-éditeurs à Einsiedeln, nous avons traduit en français le Manuel illustré de la Géographie de la Suisse que M"". Waser, professeur à Fécole normale de Schwytz, vient de publier en langue allemande. *) Nous sommes persuadé qu^un accueil bienveillant lui est réservé dans les différents établissements d'instruction publique de la Suisse française ainsi que dans les familles. Les manuels de Géographie ne font certes pas défaut dans nos écoles, mais aucun, peut-être, ne répond aussi bien aux exigences de cette branche de renseignement. La description des différentes armoiries, un petit aperçu historique de la Suisse et de chaque canton, de nombreuses et intéressantes vignettes donnent à cet ouvrage un caractère tout particulier que nous ne trouvons pas dans les autres ma- nuels de géographie. Aussi nous n'hésitons pas à déclarer que M"". Waser en élaborant ce travail, et les M. M. Benziger en l'éditant, ont rendu un grand service aux écoles de la Suisse, et nous devons leur en témoigner notre reconnaissance. Fribourg en Juin 188L J. S. -
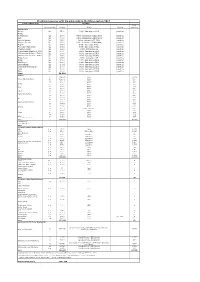
Stocking Measures with Big Salmonids in the Rhine System 2017
Stocking measures with big salmonids in the Rhine system 2017 Country/Water body Stocking smolt Kind and stage Number Origin Marking equivalent Switzerland Wiese Lp 3500 Petite Camargue B1K3 genetics Rhine Riehenteich Lp 1.000 Petite Camargue K1K2K4K4a genetics Birs Lp 4.000 Petite Camargue K1K2K4K4a genetics Arisdörferbach Lp 1.500 Petite Camargue F1 Wild genetics Hintere Frenke Lp 2.500 Petite Camargue K1K2K4K4a genetics Ergolz Lp 3.500 Petite Camargue K7C1 genetics Fluebach Harbotswil Lp 1.300 Petite Camargue K7C1 genetics Magdenerbach Lp 3.900 Petite Camargue K5 genetics Möhlinbach (Bachtele, Möhlin) Lp 600 Petite Camargue B7B8 genetics Möhlinbach (Möhlin / Zeiningen) Lp 2.000 Petite Camargue B7B8 genetics Möhlinbach (Zuzgen, Hellikon) Lp 3.500 Petite Camargue B7B8 genetics Etzgerbach Lp 4.500 Petite Camargue K5 genetics Rhine Lp 1.000 Petite Camargue B2K6 genetics Old Rhine Lp 2.500 Petite Camargue B2K6 genetics Bachtalbach Lp 1.000 Petite Camargue B2K6 genetics Inland canal Klingnau Lp 1.000 Petite Camargue B2K6 genetics Surb Lp 1.000 Petite Camargue B2K6 genetics Bünz Lp 1.000 Petite Camargue B2K6 genetics Sum 39.300 France L0 269.147 Allier 13457 Rhein (Alt-/Restrhein) L0 142.000 Rhine 7100 La 31.500 Rhine 3150 L0 5.000 Rhine 250 Doller La 21.900 Rhine 2190 L0 2.500 Rhine 125 Thur La 12.000 Rhine 1200 L0 2.500 Rhine 125 Lauch La 5.000 Rhine 500 Fecht und Zuflüsse L0 10.000 Rhine 500 La 39.000 Rhine 3900 L0 4.200 Rhine 210 Ill La 17.500 Rhine 1750 Giessen und Zuflüsse L0 10.000 Rhine 500 La 28.472 Rhine 2847 L0 10.500 Rhine 525 -

Karte Der Erdbebenzonen Und Geologischen Untergrundklassen
Karte der Erdbebenzonen und geologischen Untergrundklassen 350 000 KARTE DER ERDBEBENZONEN UND GEOLOGISCHEN UNTERGRUNDKLASSEN FÜR BADEN-WÜRTTEMBERG 1: für Baden-Württemberg 10° 1 : 350 000 9° BAYERN 8° HESSEN RHEINLAND- PFALZ WÜRZBUR G Die Karte der Erdbebenzonen und geologischen Untergrundklassen für Baden- Mainz- Groß- Main-Spessart g Wertheim n Württemberg bezieht sich auf DIN 4149:2005-04 "Bauten in deutschen Darmstadt- li Gerau m Bingen m Main Kitzingen – Lastannahmen, Bemessung und Ausführung üblicher Freudenberg Erdbebengebieten Mü Dieburg Ta Hochbauten", herausgegeben vom DIN Deutsches Institut für Normung e.V.; ub Kitzingen EIN er Burggrafenstr. 6, 10787 Berlin. RH Alzey-Worms Miltenberg itz Die Erdbebenzonen beruhen auf der Berechnung der Erdbebengefährdung auf Weschn Odenwaldkreis Main dem Niveau einer Nicht-Überschreitenswahrscheinlichkeit von 90 % innerhalb Külsheim Werbach Großrinderfeld Erbach Würzburg von 50 Jahren für nachfolgend angegebene Intensitätswerte (EMS-Skala): Heppenheim Mud Pfrimm Bergst(Bergstraraßeß) e Miltenberg Gebiet außerhalb von Erdbebenzonen Donners- WORMS Tauberbischofsheim Königheim Grünsfeld Wittighausen Gebiet sehr geringer seismischer Gefährdung, in dem gemäß Laudenbach Hardheim des zugrunde gelegten Gefährdungsniveaus rechnerisch die bergkreis Höpfingen Hemsbach Main- Intensität 6 nicht erreicht wird Walldürn zu Golla Bad ch Aisch Lauda- Mergentheim Erdbebenzone 0 Weinheim Königshofen Neustadt Gebiet, in dem gemäß des zugrunde gelegten Gefährdungsniveaus Tauber-Kreis Mudau rechnerisch die Intensitäten 6 bis < 6,5 zu erwarten sind FRANKENTHAL Buchen (Odenwald) (Pfalz) Heddes-S a. d. Aisch- Erdbebenzone 1 heim Ahorn RHirschberg zu Igersheim Gebiet, in dem gemäß des zugrunde gelegten Gefährdungsniveaus an der Bergstraße Eberbach Bad MANNHEIM Heiligkreuz- S c Ilves- steinach heff Boxberg Mergentheim rechnerisch die Intensitäten 6,5 bis < 7 zu erwarten sind Ladenburg lenz heim Schriesheim Heddesbach Weikersheim Bad Windsheim LUDWIGSHAFEN Eberbach Creglingen Wilhelmsfeld Laxb Rosenberg Erdbebenzone 2 a. -

Council CNL(14)23 Annual Progress Report on Actions Taken
Agenda Item 6.1 For Information Council CNL(14)23 Annual Progress Report on Actions Taken Under Implementation Plans for the Calendar Year 2013 EU – Germany CNL(14)23 Annual Progress Report on Actions taken under Implementation Plans for the Calendar Year 2013 The primary purposes of the Annual Progress Reports are to provide details of: • any changes to the management regime for salmon and consequent changes to the Implementation Plan; • actions that have been taken under the Implementation Plan in the previous year; • significant changes to the status of stocks, and a report on catches; and • actions taken in accordance with the provisions of the Convention These reports will be reviewed by the Council. Please complete this form and return it to the Secretariat by 1 April 2014. The annual report 2013 is structured according to the catchments of the rivers Rhine, Ems, Weser and Elbe. Party: European Union Jurisdiction/Region: Germany 1: Changes to the Implementation Plan 1.1 Describe any proposed revisions to the Implementation Plan and, where appropriate, provide a revised plan. Item 3.3 - Provide an update on progress against actions relating to Aquaculture, Introductions and Transfers and Transgenics (section 4.8 of the Implementation Plan) - has been supplemented by a new measure (A2). 1.2 Describe any major new initiatives or achievements for salmon conservation and management that you wish to highlight. Rhine ICPR The 15th Conference of Rhine Ministers held on 28th October 2013 in Basel has agreed on the following points for the rebuilding of a self-sustainable salmon population in the Rhine system in its Communiqué of Ministers (www.iksr.org / International Cooperation / Conferences of Ministers): - Salmon stocking can be reduced step by step in parts of the River Sieg system in the lower reaches of the Rhine, even though such stocking measures on the long run remain absolutely essential in the upper reaches of the Rhine, in order to increase the number of returnees and to enhance the carefully starting natural reproduction. -

Strategien Zur Wiedereinbürgerung Des Atlantischen Lachses
Restocking – Current and future practices Experience in Germany, success and failure Presentation by: Dr. Jörg Schneider, BFS Frankfurt, Germany Contents • The donor strains • Survival rates, growth and densities as indicators • Natural reproduction as evidence for success - suitability of habitat - ability of the source • Return rate as evidence for success • Genetics and quality of stocking material as evidence for success • Known and unknown factors responsible for failure - barriers - mortality during downstream migration - poaching - ship propellers - mortality at sea • Trends and conclusion Criteria for the selection of a donor-strain • Geographic (and genetic) distance to the donor stream • Spawning time of the donor stock • Length of donor river • Timing of return of the donor stock yesterdays environment dictates • Availability of the source tomorrows adaptations (G. de LEANIZ) • Health status and restrictions In 2003/2004 the strategy of introducing mixed stocks in single tributaries was abandoned in favour of using the swedish Ätran strain (Middle Rhine) and french Allier (Upper Rhine) only. Transplanted strains keep their inherited spawning time in the new environment for many generations - spawning time is stock specific. The timing of reproduction ensures optimal timing of hatching and initial feeding for the offspring (Heggberget 1988) and is of selective importance Spawning time of non-native stocks in river Gudenau (Denmark) (G. Holdensgaard, DCV, unpublished data) and spawning time of the extirpated Sieg salmon (hist. records) A common garden experiment - spawning period (lines) and peak-spawning (boxes) of five introduced (= allochthonous) stocks returning to river Gudenau (Denmark) (n= 443) => the Ätran strain demonstrates the closest consistency with the ancient Sieg strain (Middle Rhine). -

Débits Résiduels Convenables -- Comment Les Déterminer?
L'environnement pratique INSTRUCTIONS Débits résiduels convenables -- Comment les déterminer? 2000 Office fédéral de l'environne- ment, des forêts et du paysage (OFEFP) L'environnement pratique INSTRUCTIONS Débits résiduels convenables -- Comment les déterminer? 2000 Publié par l'Office fédéral de l'environnement, des forêts et du paysage (OFEFP) Auteurs R. Estoppey, OFEFP, Berne Dr. B. Kiefer, Kiefer & Partners AG, Zurich M. Kummer, OFEFP, Berne S. Lagger, OFEFP, Berne H. Aschwanden, SHGN, Berne (chap. 7) Traduction B. Bressoud, Ardon Commande Office fédéral de l'environnement, des forêts et du paysage Documentation 3003 Berne Fax + 41 (0)31 324 02 16 E-mail: [email protected] Internet: http://www.admin.ch/buwal/publikat/f/ Numéro de commande VU-2701-F © OFEFP 2000 Table des matières 3 TABLE DES MATIÈRES $9$17352326 ,1752'8&7,21 1.1 GÉNÉRALITÉS..................................................................................................................................7 1.2 OBJECTIF ET PRINCIPES DE LA FIXATION DE DÉBITS RÉSIDUELS CONVENABLES ......8 1.3 BASES LÉGALES..............................................................................................................................8 1.4 LE SYSTÈME DES DISPOSITIONS DE LA LEAUX SUR LES DÉBITS RÉSIDUELS................10 /¶$8725,6$7,21'(35e/Ê9(0(17'¶($8 2.1 PRÉLÈVEMENTS D’EAU SOUMIS À AUTORISATION ............................................................13 2.2 CONDITIONS POUR L’OCTROI DE L’AUTORISATION ...........................................................19 -

Internationally Coordinated Management Plan 2015 for the International River Basin District of the Rhine
Internationally Coordinated Management Plan 2015 for the International River Basin District of the Rhine (Part A = Overriding Part) December 2015 Imprint Joint report of The Republic of Italy, The Principality of Liechtenstein, The Federal Republic of Austria, The Federal Republic of Germany, The Republic of France, The Grand Duchy of Luxemburg, The Kingdom of Belgium, The Kingdom of the Netherlands With the cooperation of the Swiss Confederation Data sources Competent Authorities in the Rhine river basin district Coordination Rhine Coordination Committee in cooperation with the International Commission for the Protection of the Rhine (ICPR) Drafting of maps Federal Institute of Hydrology, Koblenz, Germany Publisher: International Commission for the Protection of the Rhine (ICPR) Kaiserin-Augusta-Anlagen 15, D 56068 Koblenz P.O. box 20 02 53, D 56002 Koblenz Telephone +49-(0)261-94252-0, Fax +49-(0)261-94252-52 Email: [email protected] www.iksr.org Translation: Karin Wehner ISBN 978-3-941994-72-0 © IKSR-CIPR-ICBR 2015 IKSR CIPR ICBR Bewirtschaftungsplan 2015 IFGE Rhein Contents Introduction .................................................................................. 6 1. General description .............................................................. 8 1.1 Surface water bodies in the IRBD Rhine ................................................. 11 1.2 Groundwater ...................................................................................... 12 2. Human activities and stresses .......................................... -

Case Study Rhine
International Commission for the Hydrology of the Rhine Basin Erosion, Transport and Deposition of Sediment - Case Study Rhine - Edited by: Manfred Spreafico Christoph Lehmann National coordinators: Alessandro Grasso, Switzerland Emil Gölz, Germany Wilfried ten Brinke, The Netherlands With contributions from: Jos Brils Martin Keller Emiel van Velzen Schälchli, Abegg & Hunzinger Hunziker, Zarn & Partner Contribution to the International Sediment Initiative of UNESCO/IHP Report no II-20 of the CHR International Commission for the Hydrology of the Rhine Basin Erosion, Transport and Deposition of Sediment - Case Study Rhine - Edited by: Manfred Spreafico Christoph Lehmann National coordinators: Alessandro Grasso, Switzerland Emil Gölz, Germany Wilfried ten Brinke, The Netherlands With contributions from: Jos Brils Martin Keller Emiel van Velzen Schälchli, Abegg & Hunzinger Hunziker, Zarn & Partner Contribution to the International Sediment Initiative of UNESCO/IHP Report no II-20 of the CHR © 2009, KHR/CHR ISBN 978-90-70980-34-4 Preface „Erosion, transport and deposition of sediment“ Case Study Rhine ________________________________________ Erosion, transport and deposition of sediment have significant economic, environmental and social impacts in large river basins. The International Sediment Initiative (ISI) of UNESCO provides with its projects an important contribution to sustainable sediment and water management in river basins. With the processing of exemplary case studies from large river basins good examples of sediment management prac- tices have been prepared and successful strategies and procedures will be made accessible to experts from other river basins. The CHR produced the “Case Study Rhine” in the framework of ISI. Sediment experts of the Rhine riparian states of Switzerland, Austria, Germany and The Netherlands have implemented their experiences in this publication. -

Spatial Patterns of Frequent Floods in Switzerland
Hydrological Sciences Journal ISSN: 0262-6667 (Print) 2150-3435 (Online) Journal homepage: http://www.tandfonline.com/loi/thsj20 Spatial patterns of frequent floods in Switzerland Klaus Schneeberger, Ole Rössler & Rolf Weingartner To cite this article: Klaus Schneeberger, Ole Rössler & Rolf Weingartner (2018): Spatial patterns of frequent floods in Switzerland, Hydrological Sciences Journal To link to this article: https://doi.org/10.1080/02626667.2018.1450505 Accepted author version posted online: 29 Mar 2018. Submit your article to this journal View related articles View Crossmark data | downloaded: 6.1.2020 https://doi.org/10.7892/boris.114004 source: Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=thsj20 Spatial patterns of frequent floods in Switzerland Klaus Schneebergera,b,c, Ole Rösslerb, Rolf Weingartnerb,c aalpS - Centre for Climate Change Adaptation, Grabenweg 68, AT-6020 Innsbruck, Austria bInstitute of Geography, Hydrology Group, University of Bern, Hallerstrasse 12, CH- 3012 Bern, Switzerland cOeschger Centre for Climate Change Research, Bern, Switzerland Contact: [email protected] Abstract This study investigates the spatial dependence of high and extreme streamflows in Switzerland across different scales. First, using 56 runoff time series from Swiss rivers, we determined the average length of high-streamflow events for different levels of extremeness. Second, a dependence measure that expressed the probability that streamflow peaks would meet or exceed streamflow peaks at a conditioning site was used to describe and map the spatial extent of joint streamflow-peak occurrences across Switzerland. Third, we analysed the spatial patterns of jointly occurring high streamflows using cluster analysis to identify groups that react similarly in terms of flood frequency at different sites.