United States Patent Office Patented Aug
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Degradation of Cyclohexane and Cyclohexanone by Bacillus Lentus Strain LP32
Vol. 12(47), pp. 6632-6635, 20 November, 2013 DOI: 10.5897/AJB2013.13137 ISSN 1684-5315 ©2013 Academic Journals African Journal of Biotechnology http://www.academicjournals.org/AJB Full Length Research Paper Degradation of cyclohexane and cyclohexanone by Bacillus lentus strain LP32 Bolanle O. Opere, Oluwafemi S. Obayori* and Adebanji A. Raji Department of Microbiology, Lagos State University, Ojo, Lagos, Nigeria. Accepted 10 October, 2013 A Gram-positive bacterium, Bacillus lentus LP32, originally isolated on the basis of its ability to utilise pyrene as sole source of carbon was found to be able to grow luxuriantly on alicyclic compounds as sole substrates. It showed poor growth on anthracene, naphthalene, 1-naphthol and phenanthrene. Growth rate on cyclohexane was 1.32 d-1, while doubling time was 0.76 d. The corresponding values for growth on cyclohexanone were 0.77 d-1 and 1.29 d, respectively. Within 10 days, the amount of cyclohexane in culture reduced from 317.62 to 102.55 mgl-1, then to 23.04 mgl-1 on day 18. On cyclohexanone, substrate concentration decreased from 287.56 mgl-1 to 101.66 mgl-1 in 10 days before declining to 24.21 mgl-1 on day 18. The rate of degradation when growing on cyclohexane was 23.50 mgl-1d-1 in the first 10 days and 9.93 mgl-1d-1 between day 10 and day 18, with 67.71% degradation in 10 days and overall percentage degradation of 92.43%. On cyclohexanone, the corresponding values were 18.59 and 9.68 mg l-1d-1 as well as 64.65 and 91.58%, respectively. -
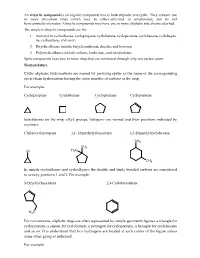
Nomenclature Cyclic Aliphatic Hydrocarbons Are Named By
An alicyclic compound is an organic compound that is both aliphatic and cyclic. They contain one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached. The simplest alicyclic compounds are the 1. monocyclic cycloalkanes: cyclopropane, cyclobutane, cyclopentane, cyclohexane, cyclohepta ne, cyclooctane, and so on. 2. Bicyclic alkanes include bicycloundecane, decalin, and housane. 3. Polycyclic alkanes include cubane, basketane, and tetrahedrane. Spiro compounds have two or more rings that are connected through only one carbon atom. Nomenclature Cyclic aliphatic hydrocarbons are named by prefixing cyclo- to the name of the corresponding open-chain hydrocarbon having the same number of carbons as the ring. For example: Cyclopropane Cyclobutane Cyclopentane Cyclopentene Substituents on the ring- alkyl, groups, halogens- are named and their positions indicated by numbers. Chlorocyclopropane 1,1- Dimethylyclopentane 1,3-Dimethylcyclohexane CH3 CH3 Cl H3C CH3 In simple cycloalkenes and cycloalkynes the double and triply bonded carbons are considered to occupy positions 1 and 2. For example: 3-Ethylcyclopentene 1,3-Cyclohexadiene H3C For convenience, aliphatic rings are often represented by simple geometric figures: a triangle for cyclopropane, a square for cyclobutane, a pentagon for cyclopentane, a hexagon for cyclohexane and so on. It is understood that two hydrogens are located at each corner of the figure unless some other group is indicated. For example H3C cyclopentane 3-Ethylcyclopentene 1,3-Cyclopentadiene CH3 CH3 Cl CH Cyclohexane 3 1,3-Dimethylcyclohexane 2- Chloro-1-methylcyclohexane As usual alcohols are given the ending –ol, which takes priority over –ene and appears last in the name. -

Tamilnadu SSLC Science Lesson 11 – One Marks
www.usefuldesk.com Tamilnadu SSLC Science Lesson 11 – One Marks Choose the correct answer: 1. The molecular formula of an open chain organic compound is C3H6. The class of the compound is Alkane Alkene Alkyne Alcohol 2. The IUPAC name of an organic compound is 3 – Methyl butan – 1 – ol. What type compound it is? Aldehyde Carboxylic acid Ketone Alcohol 3. The secondary suffix used in IUPAC nomenclature of an aldehyde is -ol oic acid -al -one 4. Which of the following pairs can be the successive members of a homologous series? C3H8 and C4H10 C2H2 and C2H4 CH4 and C3H6 C2H5OH and C4H8OH 5. C2H5OH + 3O2 -> 2CO2 + 3H2O is a Reduction of ethanol Combustion of ethanol Oxidation of ethanoic acid Oxidation of ethanol 6. Rectified spirit is an aqueous solution which contains about _________ of ethanol. 95.5 % Website: www.usefuldesk.comFacebook: www.facebook.com/usefuldeskE-mail: [email protected] www.usefuldesk.com 75.5 % 55.5 % 45.5 % 7. Which of the following are used as anaesthetics? Carboxylic acids Ethers Esters Aldehydes 8. TFM in soap represents _______ content in soap. Mineral Vitamin Fatty acid Carbohydrate 9. Which of the following statements is wrong about detergents? It is a sodium salt of long chain fatty acids. It is sodium salts of sulphonic acids The ionic part in a detergent is –SO3 –Na+ It is effective even in hard water. Fill in the blanks: 1. An atom or a group of atoms which is responsible for chemical characteristics of an organic compound is called _______ Functional group 2. The general molecular formula of alkynes is ______ CnH2n-2 3. -

Facts on File DICTIONARY of CHEMISTRY
The Facts On File DICTIONARY of CHEMISTRY The Facts On File DICTIONARY of CHEMISTRY Fourth Edition Edited by John Daintith The Facts On File Dictionary of Chemistry Fourth Edition Copyright © 2005, 1999 by Market House Books Ltd All rights reserved. No part of this book may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage or retrieval systems, without permission in writing from the publisher. For information contact: Facts On File, Inc. 132 West 31st Street New York NY 10001 For Library of Congress Cataloging-in-Publication Data, please contact Facts On File, Inc. ISBN 0-8160-5649-8 Facts On File books are available at special discounts when purchased in bulk quantities for businesses, associations, institutions, or sales promotions. Please call our Special Sales Department in New York at (212) 967-8800 or (800) 322-8755. You can find Facts On File on the World Wide Web at http://www.factsonfile.com Compiled and typeset by Market House Books Ltd, Aylesbury, UK Printed in the United States of America MP PKG 10 9 8 7 6 5 4 3 2 1 This book is printed on acid-free paper. PREFACE This dictionary is one of a series designed for use in schools. It is intended for stu- dents of chemistry, but we hope that it will also be helpful to other science students and to anyone interested in science. Facts On File also publishes dictionaries in a variety of disciplines, including biology, physics, mathematics, forensic science, weather and climate, marine science, and space and astronomy. -

UNITED STATES PATENT OFFICE 2,349,232 MANUFACTURE of ALICYCLC COMPOUNDS Lloydi M
Patented May 16, 1944 2,349,232 UNITED STATES PATENT OFFICE 2,349,232 MANUFACTURE OF ALICYCLC COMPOUNDS Lloydi M. Joshe, Hyattsville, Md., assignor to the United States of America as represented by Claude R. Wickard, Secretary of Agricult2 re, and his successors in office No Drawing. Applieation October 16, 194A, Seria No. 4:5,263 4. Caians. (CA. 260-666) (Granted under the act of March 3, 1883, as amended April 30, 1928; 3 (0. (G. 57) This application is made under the act of and that greater yields may be obtained with March 3, 1883, as amended by the act of April other compounds at higher temperatures. The 30, 1928, and the invention herein described, if temperature used, however, in any case should patented, may be manufactured and used by or not be so high as to have a destructive effect upon for the Government of the United States of the final product being formed. The pressures America, for governmental purposes Without the used also depend a great deal upon the character payment to me of any royalty thereon. of the diene being used, as well as the vessel in This invention relates to a news process for which the reaction is carried out. Generally, manufacturing organic compounds of the ali there is not a critical upper limit with respect to cyclic class. 0 pressure and, ordinarily, pressures above about In general, the process comprises reacting 1,000 pounds per Square inch at room tempera ethylene with a conjugated diene, also known as ture can be used to obtain satisfactory results. -

Organic Chemistry – Some Basic Principles & Techniques
ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES ORGANIC What does the word ‘organic’ means ? Organic compounds are derived from living organisms. ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES ORGANIC CHEMISTRY It is the study of compounds extracted from living organisms. ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES Organic Compounds Contains carbon It shows catenation. (Ability of carbon atoms to combine, forming long chains) Organic Chemistry Carbon Chemistry ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES Introduction What are organic compounds ? Compounds which OrganicOrganic Inorganic contain carbon compoundscompounds compounds So, the correct CH NaCl classification of the 4C CH4 NaCl But …. C6HC12CO6H6 12O6 HClHCl compounds is H2SO4 C2HC5OH2H5 OH H2SO4 NaOH COC NaOH CO, CO2, metal carbonates and 2 CO COC 2 bicarbonates, cyanates are CCO inorganic compounds though Na2CCO3 Na CCO 2 3 they contains carbon, because these NaHCCO3 NaHCO 3 compounds do not follow the NHCCNO 4 NH4CNO properties of organic compounds. ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES Organic Inorganic Compounds Compounds Extracted from Study of non–living living organism organism such as e.g.- Sugar , Rocks, Urea , etc. minerals, etc. ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES In 1815 Berzelius Proposed that a vital force was responsible for the formation of organic compounds ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES In 1828 Wohler Synthesized first organic compound urea from inorganic compound. So, Vital force theory was disproved by Wohler NH4CNO H2N – C – NH2 (ammonium O cyanate) (Urea) (Inorganic) (Organic) ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES In 1845 Kolbe Synthesized acetic acid from its elements. ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES & TECHNIQUES In 1856 Berthelot Synthesized methane. -

United States Patent '0 Rice Patented Aug
1 3,597,403‘ United States Patent '0 rice Patented Aug. 3, 197]]. l 2 halides, (B) at least one compound selected from a group 3,597,403 NOVEL CATALYSTS FOR THE RING-OPENING consisting of molecular oxygen, chlorine, bromine, iodine POLYMERIZATION 0F UNSATIURATED ALllCY and cyanogen halides, and (C) at least one transition CLlC COMPDUNDS metal compound selected from the group consisting of Eilert A. Ofstead, Cuyahoga Falls, Ohio, assignor to The tungsten and molybdenum complexes corresponding to the Goodyear Tire & Rubber Company, Akron, Ohio formula M (CO),R Where M is tungsten and molybdenum, No Drawing. Filed Oct. 27, 1969, Ser. No. 869,909 C is carbon, 0 is oxygen and R is an unsaturated hydro lint. Cl. C08f 7/02, 15/04 carbon compound having at least two non-conjugated car US. Cl. 260-881 8 Claims bon-to-carbon double bonds and where R is attached to 10 the transition metal by coordination through two carbon to-carbon double bonds. ABSTRACT OF THE DISCLOSURE Representative examples of the compounds useful as the There is disclosed a process for the ring-opening polym (A) catalyst component include alkylaluminum dihalides erization of certain unsaturated alicyclic compounds such as ethylaluminum dichloride, isobutylaluminum di which comprises subjecting said unsaturated alicyclic com 15 chloride, propylaluminum dichloride, ethylaluminum di pounds to a catalyst system comprising (A) at least one bromide, ethylaluminum diiodide and the like; alkylalu compound selected from a group consisting of alkylalu minum sesquihalides such as ethylaluminum sesquichlo minum dihalides, alkylaluminum sesquihalides and alu ride, methylaluminum sesquichloride, methylaluminum minum halides, (B) at least one compound selected from sesq-uibromide and the like; and aluminum halides such the group consisting of molecular oxygen, chlorine, bro 20 as aluminum chloride, aluminum bromide and the like. -

Journal of Polymer Science Part A-1 Polymer Chemistry 1967 Volume.5
JOURNAL OF POLYMER SCIENCE: PART A-l VOL. 5, 2209-2217 (1967) Ring-Opening Polymerization of Unsaturated Alicyclic Compounds NISSIM CALDERON, EILERT A. OFSTEAD, and W. ALLEN JUDY, Research Division, The Goodyear Tire and Rubber Company, Akron, Ohio 44316 Synopsis The ring-opening polymerizations of cyclooctene, cyclododecene, 1,5-cyclooctadiene, 1,5,9-cyelododecatriene, 3-methylcyclooctene, and 3-phenylcyclooctene have been car ried out by using a two-component catalyst system composed of ethylaluminum di chloride and tungsten hexachloride. N M R and infrared analyses of the respective poly mers indicate structures which are consistent with a ring-cleavage mode of propagation. No evidence for double-bond shifts or transannular reactions during the polymerizations of 1,5-cyclooctadiene, 1,5,9-cyclododecatriene, 3-methylcyclooctene, and 3-phenyl- cyclooctene was found. The polymerizability of substituted, unsaturated, medium sized alicyclic monomers suggests a convenient method for synthesis of certain perfectly alternating terpolymers. Since polymerizations occurred rapidly with little evolution of heat, it was concluded that entropy is a substantial contributor to the free energy of the ring-opening polymerization of medium-sized, unsaturated alicyclic mono mers. INTRODUCTION The polymerization of cyclopentene by a ring-opening mechanism, lead ing to the formation of a solid, elastomeric polymer, was first disclosed by Eleuterio.1 A linear, unsaturated repeat unit having the formula [—CH2—• CH2— C H =C H — CH2—] was suggested, where -

¶F&Kl?AZE Ndmac
DISTRIBUTION LISP PDR ME&T 63-0970 D/596,6C1T D/5140, FB68 D/5143, FB66 Dr. R. Paton W .J. Costas M . Francis M. Mogg D/539, AC1O D/5614, n66 R. Schmued . Buckles J.A. Lieb W D/596, SSii R .P. Jevett M .C. Shoemaker F . 'Ii' J. Wooten D/031, SS32 D/539, A129 R. Johan W.T.- McFarlen C . Winzer W. Kappen R. McKovan D/539, HC92 3. Levis D/539, BA33 F . Schuler D/539, 33T R . Movers J. Rosengard D/539, BA71 3. Becker J J D/539, HC82 - K. Kirkhan ¶f&kl?AZE ~~}12 CI-~ oti NDMAc (14 ITZo5OD (ThYtAfl*Nt3 II0Imuimiiimmimiiiuumiiuiuu BNA00834964 HDMSE00609163 Internal Letter ® Rockwell International Date. November 28, 1983 No . MEAT 83-097 0 FROM : t Natne orQantraa °n . rnremal Andress . Pnonel TO rNaTe Orpan.: .'cn, lnleraar AU essl . Michael Francis Norma Fujikawa, Manager Environmental Task Team . SSFL Analytical Chemistry . 0/541, FB 12 . 0/539-169, SS 1 1 5448 ) Subject : . Literature Search Findings on N-Nitrosodimethylamine (NDMA At your request , the toxic levels, the general abundance of the compound, and the analytical methods for the determination of N-nitrosodimethylamine have been reviewed . ABSTRACT It is now generally agreed that N-nitroso compounds are among the most potent chemical carcinogens known . The U .S . Department of Health, Human .4 Services , and Public Health ~ervice has reported that a concentration of 1 10- grams /liter, or 0 .0000014 ppm) of parts per trillion (1 .4x e N-nitrosodimethylamine ( NDMA ) is estimated to limit cancer risk to on in a million . -

Tamilnadu Board Class 11 Chemistry Chapter 11
Unit 11 Fundamentals of Organic Chemistry Learning Objectives After studying this unit students will be able to • understand the reason for the tetra valency of carbon and shapes of organic molecules • classify the organic compounds • name the organic compounds according to IUPAC nomenclature and derive the structure from the IUPAC name • describe various types of isomerism Friedrich Wöhler, was a German pioneer in organic • explain the principles of detection and estimation chemistry. He is best known of elements in organic compounds for his synthesis of urea an the organic compound from • describe various techniques used in the the inorganic compound, purification of organic compounds ammonium cyanate. This finding went against the Introduction mainstream theory of that time called vitalism which Organic chemistry is the study of compounds stated that organic matter of carbon. Carbon has a tendency to form more possessed a special force compounds with itself and other atoms (H, O, N, S or vital force inherent to and halogens) than any other elements. The tendency all living things. He is also of an atom to form a chain of bonds with the atoms the first to isolate several of the same element is called catenation. The high chemical elements. He is the strength of C-C bond is responsible for its catenation discoverer of the element property. aluminum and also the co-discoverer of yttrium, The word 'organic' means 'derived from living beryllium, and titanium. organisms'. Organic compounds were thought to be found only in living things. Cell the basic unit of living things, consumes, creates and consists of mainly organic compounds. -

APPENDIXES APPENDIX 1 Reference Books and Journals on Catalysis
APPENDIXES APPENDIX 1 Reference Books and Journals on Catalysis 1. GENERAL CONCEPTS IN CATALYSTS 1.1. Afansiev, V. A., and Zarkov, G. E., In the Realm of Catalysis, Mir Publishers, Moscow (1979). 1.2. Ashmore, P. G., Catalysis and Inhibition of Chemical Reactions, Butterworth and Co., London (1963). 1.3. Anderson, J. R., Structure of Metallic Catalysts, Academic Press, New York (1975). 1.4. Balandin, A. A. (Editor), Catalysis and Chemical Kinetics, Academic Press, New York (1964). 1.5. Bartok, M. (Editor), Stereochemistry of Heterogeneous Metal Catalysts, John Wiley, New York (1985). 1.6. Basalo, F., and Burwell Jr., R. L. (Editors), Catalysis: Progress in Research, Plenum Press, New York (1973). 1.7. Bell, A. T., and Hegedus, L. L. (Editors), Catalysis Under Transient Conditions, ACS Symposium Series No. 178, American Chemical Society, Washington, D.C (1982). 1.8. Bell, R. P., Acid-Base Catalysis, The Clarendon Press, Oxford (1941). 1.9. Berkman, S., Morrell, J. C, and Egloff, G., Catalysis, Inorganic and Organic, Reinhold Publishing Corp., New York (1940). 1.10. Boldyrev, V. V., Bulens, M., and Delmon, B., The Control of the Reactivity of Solids, Elsevier, Amsterdam (1979). l.ll. Bond, G. C, Catalysis by Metals, Academic Press, New York (1962). 1.12. Bond, G. C, The Principles of Catalysis-Monograph for Teachers # 7, Royal Institute of Chemistry, London (1968). l.I3. Bond, G. C, Heterogeneous Catalysis, Principles and Applications, Clarenden Press, Oxford (1974). 1.14. Bosnich, B., Asymmetric Catalysis, Martinus Nijhoff Publishers, The Hague (1986). 1.15. Bornelli, Delmon, B., and Derouane, E. (Editors), Surface Properties and Catalysis by Non-Metals, D. -

Rodd's Chemistry O F Carbon Compound S
Supplements to the 2nd Edition (Editor S. Coffey) of RODD'S CHEMISTRY O F CARBON COMPOUND S A modern comprehensive treatise Edited by M. F. ANSELL Ph.D., D.Sc. (London), F.R.I.C. Department of Chemistry, Queen Mary College , University of London (Great Britain) Supplement t o VOLUME II ALICYCLIC COMPOUND S Part A : Monocarböcyclic compounds to and including five ring atom s Part B: Six- and higher-membered monocyclic compounds VOLUME II A B Alicyclic Compounds ; Monocarbocyclic Compounds to and including Five Rings Atoms ; Six- and Higher-Membered Monocyclic Compounds . Preface vi i Official publications ; Scientific Journals and periodicals xiv List of common abbreviations and symbols used x v Chapter 1 . Introductio n by MARTIN F . ANSEL L 1 . Pericyclic reactions . 1 a. Generalised Woodward-Hoffmann rule for pericyclic reaction . 6 b. Theoretical basis of Woodward-Hoffmann rules . 7 2 . Stereochemistry and conformational analysis 8 Chapter 2 . The Cyclopropane Group by P . H . BOYL E 1 . Preparative methods 9 a. Methods based on 1,3-cyclisation 9 b. Methods based on ring contraction . 1 2 c. Methods based on carbene addition . 1 4 (i) Halocarbenes, 14 - (ii) Other carbenoid reactions, 16 - 2 . Saturated hydrocarbons . 1 8 3 . Unsaturated hydrocarbons . 2 0 4 . Halogen derivatives of cyclopropane 2 7 5 . Organometallic compounds derived from cyclopropanes 3 0 6 . Alcohols derived from cyclopropanes 3 1 7 . Sulphur derivatives of cyclopropanes 3 3 8 . Nitrocyclopropanes . 3 4 9 . Amines and related compounds derived from cyclopropanes . 3 4 10 . Aldehydes and ketones derived from cyclopropanes 3 6 11 . Cyclopropane- and cyclopropene-carboxylic acids 4 3 12 .